Contents
Think electricity only comes from giant power plants? Think again! Every battery you use is actually a mini galvanic cell powered by a chemical reaction. In this article on the Tech4Ultra Electrical website, we’ll break down the mystery of the voltaic cell, showing how electrons flow from the anode to the cathode, and why a salt bridge is absolutely essential. You’ll walk away with a clear understanding of how redox reactions generate cell voltage inside an electrochemical cell, using zinc and copper plates as a real-world example. Whether you’re a student or a curious mind, this guide will make electrochemistry click.
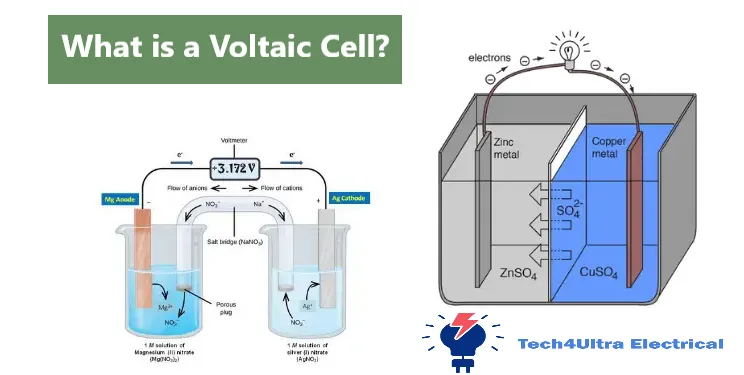
Basic Components of a Voltaic Cell
Electrodes: Anode and Cathode
Every voltaic cell starts with two key components: the electrodes. The anode is where oxidation takes place — electrons are lost here and travel through an external circuit. The cathode, on the other hand, is where reduction happens — it gains those same traveling electrons. This movement of charge is what powers devices connected to the cell. Each electrode is typically made of a metal like zinc or copper, selected based on their position in the activity series and their tendency to undergo redox reactions.
Electrolyte Solution
The electrolyte solution allows ions to move inside each half of the electrochemical cell. It surrounds the electrodes and completes the internal part of the circuit. For example, in a zinc-copper galvanic cell, zinc sulfate and copper sulfate solutions are used to stabilize ion exchange.
External Wire and Voltmeter
An external wire connects the two electrodes, enabling the electrons flow from the anode to the cathode. A voltmeter placed along this path measures the cell voltage — essentially the electrical potential difference generated by the redox reaction.
Salt Bridge (in modern designs)
The salt bridge is crucial in maintaining charge balance between the two half-cells. It allows the flow of ions to neutralize the charge buildup caused by the electrons flow, ensuring the reaction continues smoothly.
Read Also: Plum Pudding Model Explained: Thomson’s Early Atomic Theory
Working Principle of a Voltaic Cell
Role of Dissimilar Metals
At the heart of a functioning voltaic cell lies the use of two dissimilar metals — each with different tendencies to lose or gain electrons. When these metals, like zinc and copper plates, are placed in separate solutions of their respective ions, they set the stage for a spontaneous redox reaction. Zinc, being more reactive, serves as the anode and tends to lose electrons more readily than copper.
Flow of Electrons from Anode to Cathode
The electrons flow begins at the anode, where zinc atoms are oxidized into Zn²⁺ ions, releasing electrons into the external circuit. These electrons travel through a wire, passing through a voltmeter which measures the cell voltage, and continue their journey to the cathode, where they reduce Cu²⁺ ions into solid copper. This electron movement is what we harness as electric current.
Redox Reaction Explanation
The core of the process is the redox reaction — simultaneous oxidation and reduction. At the anode, oxidation occurs (Zn → Zn²⁺ + 2e⁻). At the cathode, reduction takes place (Cu²⁺ + 2e⁻ → Cu). This exchange of electrons is what keeps the cell active and allows electrical energy to be continuously produced as long as the reactants last.
Electrical Energy Generation
This movement of electrons and the accompanying ion transfer through the salt bridge creates a flow of electrical charge — what we call electricity. It’s this conversion of chemical energy into electrical energy that makes the electrochemical cell useful in countless devices, from simple batteries to complex energy storage systems.
Step-by-Step Example: Zinc-Copper Voltaic Cell
Detailed Breakdown of Reactions
Let’s walk through a real example — the classic zinc and copper plates in a voltaic cell. This setup is widely used to demonstrate the fundamentals of electrochemical cells. Zinc metal is placed in a zinc sulfate (ZnSO₄) solution and copper metal in a copper sulfate (CuSO₄) solution. The two are connected by a wire and a salt bridge to allow ion movement.
Oxidation at Zinc Electrode
At the anode, zinc undergoes oxidation. It loses electrons and becomes Zn²⁺ ions, which dissolve into the solution. This can be expressed as:
Zn (s) → Zn²⁺ (aq) + 2e⁻
This reaction starts the electrons flow toward the other side of the circuit — the copper electrode.
Reduction at Copper Electrode
On the other end, at the cathode, Cu²⁺ ions in the solution gain the arriving electrons and get reduced to solid copper, depositing onto the electrode. The reaction is:
Cu²⁺ (aq) + 2e⁻ → Cu (s)
This is the reduction half of the redox reaction.
Net Ionic Equation
The overall half‑cell reaction for the cell becomes:
Zn (s) + Cu²⁺ (aq) → Zn²⁺ (aq) + Cu (s)
This equation summarizes the entire electrochemical cell process — a complete exchange of electrons that results in the generation of cell voltage.
Formation of Hydrogen Gas
While hydrogen gas is not typically formed in this particular zinc-copper galvanic cell, in some acidic electrolytes, hydrogen ions may gain electrons at the cathode to form H₂ gas. However, in this standard example, the primary focus is on the metal displacement and electron transfer.
Common Problems in Voltaic Cells
a. Polarization
Cause (Hydrogen Gas Buildup): One of the most frequent issues in a voltaic cell is polarization. This happens when hydrogen gas bubbles start to accumulate on the surface of the cathode, especially in cells where hydrogen ions are reduced instead of metal ions. The buildup creates a layer that blocks the electrons flow, acting like a barrier to the ongoing redox reaction.
Effects on Current: As the gas layer grows, it increases internal resistance. The result? A significant drop in cell voltage and current output. Essentially, even if the chemical reaction is happening, the electrical output becomes sluggish or stops completely.
How to Prevent It: To reduce polarization, chemical depolarizers like nitric acid or manganese dioxide are used. Another method is mechanical — physically removing gas bubbles or shaking the solution. For more stable operation, modern electrochemical cells avoid conditions where hydrogen buildup is common.
b. Local Action
Cause (Impurities in Zinc): In the anode (usually zinc), even tiny impurities like iron or copper can act as mini-electrodes, creating small galvanic spots across the surface. This causes uncontrolled local reactions known as “local action.”
Wastage of Zinc: These micro-reactions consume zinc unnecessarily, even when the cell isn’t connected to a circuit. It leads to early depletion of the electrode and reduces the overall lifespan of the galvanic cell.
Use of Amalgamated Zinc: To counter this, zinc is often treated with mercury to form a smooth amalgamated layer. This coats the zinc and neutralizes the effect of impurities, minimizing unwanted half‑cell reactions and preserving efficiency.
Voltage Generation and Electrode Potentials
Standard Electrode Potential Values
Every metal used in a voltaic cell has a tendency to gain or lose electrons. This is measured as its standard electrode potential (E°). These values are determined under standard conditions and are listed in electrochemical series tables. In a typical zinc and copper plates cell, zinc has an E° of -0.76 V and copper has +0.34 V. These values help predict which metal will serve as the anode (oxidation) and which as the cathode (reduction).
Calculation of EMF Using E° Values
The cell voltage or EMF (electromotive force) of the electrochemical cell can be calculated using the formula:
EMF = E°(cathode) – E°(anode)
So for a zinc-copper galvanic cell: EMF = 0.34 V – (–0.76 V) = 1.10 V. This voltage drives the electrons flow from the zinc electrode to the copper electrode, enabling the overall redox reaction.
Nernst Equation Brief Intro
For more advanced readers, the Nernst equation allows you to calculate EMF under non-standard conditions by accounting for ion concentrations. It shows how changes in concentration can influence the voltage output of a half‑cell reaction, making it a vital tool in real-world applications of electrochemical cells.
Types of Voltaic Cells
Daniell Cell
The classic Daniell cell is one of the earliest and most stable forms of the voltaic cell. It uses zinc and copper plates in their respective sulfate solutions, connected by a salt bridge. It reliably generates about 1.1 V and clearly demonstrates the principles of electrons flow and redox reactions.
Concentration Cell
A unique type of electrochemical cell where the electrodes are identical, but the electrolyte concentrations differ. This creates a potential difference, causing electrons to move until equilibrium is reached.
Primary vs Secondary Voltaic Cells
Primary cells (like AA batteries) are disposable — the redox reactions are irreversible. Secondary cells (like car batteries) are rechargeable and can reverse the reaction using external current.
Dry Cell vs Wet Cell Comparison
Dry cells contain a paste-like electrolyte, making them portable and leak-proof. Wet cells use liquid electrolytes and are often found in laboratory setups. Both rely on similar half‑cell reactions to produce cell voltage.
Watch Also: Planar and Non-Planar Graphs Explained with Circuit Examples
Real-World Applications of Voltaic Cells
Batteries (AA, AAA, Lead-Acid, Lithium)
Everyday batteries like AA and AAA are compact voltaic cells designed for low-power devices. Lead-acid batteries use multiple electrochemical cells to power vehicles, while lithium batteries offer high energy density for portable gadgets and electric vehicles. All rely on redox reactions and steady electrons flow from anode to cathode.
Fuel Cells
Fuel cells are advanced galvanic cells that generate electricity from a continuous fuel supply like hydrogen, producing clean energy with only water as a byproduct. Their efficiency and sustainability make them a key part of future energy systems.
Portable Electronics
Phones, laptops, and tablets all rely on miniaturized electrochemical cells. These cells convert chemical energy into electrical energy through controlled half‑cell reactions, providing stable cell voltage during operation.
Medical Devices
Devices like pacemakers and hearing aids use tiny voltaic cells that offer long-term, reliable power with minimal maintenance, showcasing how precise control over redox reactions supports life-saving technologies.
Conclusion
Voltaic cells convert chemical energy into electrical energy through spontaneous redox reactions involving electrons flow from anode to cathode. Using setups like zinc and copper plates, these reactions are harnessed to generate useful cell voltage. Understanding their principles is essential in both physics and chemistry, especially for grasping how electrochemical cells work. We also explored key issues like polarization and local action, and their solutions. From powering portable devices to life-saving medical tools, the practical applications of galvanic cells are all around us — making them one of the most impactful topics in science education.
FAQs
What is the difference between voltaic and electrolytic cells?
A voltaic cell generates electricity from a spontaneous redox reaction, while an electrolytic cell uses external electrical energy to drive a non-spontaneous reaction. In voltaic cells, electrons flow naturally from anode to cathode; in electrolytic cells, an external power source forces the electron movement.
Why does zinc lose electrons?
Zinc is more reactive than many other metals. In a galvanic cell, it serves as the anode and undergoes oxidation, releasing electrons because it has a lower standard electrode potential than metals like copper.
Can a voltaic cell be recharged?
Standard voltaic cells are usually not rechargeable. However, secondary voltaic cells, like lithium-ion or lead-acid batteries, are designed to reverse the redox reaction through external charging.
How do voltaic cells work?
They convert chemical energy to electrical energy via spontaneous redox reactions. Electrons flow from the anode (oxidation site) through an external wire to the cathode (reduction site), with a salt bridge balancing ion flow.
What is the principle and working of galvanic cell?
A galvanic cell works on the principle of redox reactions. The difference in electrode potentials between two metals drives the electrons flow, generating a measurable cell voltage.
What is a simple voltaic cell?
A simple voltaic cell consists of two dissimilar metals like zinc and copper plates in their respective ion solutions, connected by a wire and a salt bridge to complete the circuit.
What is the electrolyte in a voltaic cell?
The electrolyte is a solution that allows ion movement within each half-cell. It maintains charge neutrality during the redox reaction and supports continuous electrons flow between the electrodes.
What is the construction and working of a voltaic cell?
A voltaic cell consists of two electrodes (typically dissimilar metals like zinc and copper plates) placed in separate electrolyte solutions, connected by a salt bridge and an external wire. It works by harnessing a spontaneous redox reaction where electrons flow from the anode (oxidation) to the cathode (reduction), generating cell voltage.
What is a voltaic cell and how does it work?
A voltaic cell is a type of electrochemical cell that generates electricity from a chemical reaction. It works by transferring electrons through a wire from the anode to the cathode, powered by a redox reaction between the metals and their ions.
How does voltaic work?
The voltaic cell works by converting chemical energy into electrical energy. Through oxidation at the anode and reduction at the cathode, electrons flow externally while ions move through a salt bridge, producing usable electrical current.
What is the construction and working of electric cell?
An electric cell, including a galvanic cell, is constructed from two half-cells — each with a metal electrode and an electrolyte. The electrons flow from the more reactive metal (anode) to the less reactive one (cathode), generating energy through a controlled redox reaction.
What is the construction and working of photovoltaic cell?
A photovoltaic cell, unlike a voltaic cell, converts sunlight directly into electricity using semiconductor materials like silicon. Its construction includes a p-n junction, metal contacts, and an antireflective coating. When sunlight hits the cell, it excites electrons in the semiconductor, causing electrons flow through an external circuit — generating a cell voltage without a chemical redox reaction.
What is the working of the cell?
In a voltaic cell, the working involves a spontaneous redox reaction that causes electrons to move from the anode to the cathode. In contrast, a photovoltaic cell works by absorbing light energy to generate electron movement across a semiconductor junction, producing direct current electricity.

1 thought on “Photometry Explained: How We Measure Light the Way Humans See It”