Contents
Have you ever wondered how an invisible burst of energy could completely change our understanding of the universe? If you’ve heard of energy quanta or quantum physics but still find the concepts confusing, you’re in the right place. In this article on the Tech4Ultra Electrical website, we’ll break down the basics of quantum theory in a simple, engaging way—revealing how this revolutionary idea reshaped modern science.
The Crisis of Classical Physics
Problems Explaining Blackbody Radiation
I still remember the first time I learned about blackbody radiation. I thought, “This should be simple physics, right?” Turns out, it was the beginning of a major scientific breakdown. Classical physics just couldn’t explain why heated objects didn’t emit infinite energy at higher frequencies.
Physicists at the time used formulas like the Rayleigh-Jeans law to predict radiation behavior, and it worked well—up to a point. But when the frequency increased, the predictions went wild. The energy output climbed off the charts. That’s when things stopped making sense. This was the moment science needed more than Newton’s comfort zone. Enter the strange, counterintuitive world of quantum physics.
The Ultraviolet Catastrophe
The problem became famously known as the ultraviolet catastrophe. The idea was terrifying: if classical theory were right, every object should be glowing with deadly levels of UV light. Clearly, that wasn’t happening in real life.
This disaster pushed scientists to rethink everything. The only way out? Introducing the concept of energy quanta. Max Planck proposed that energy isn’t released smoothly, but in tiny, specific amounts—like steps, not a slide. And just like that, quantum theory was born, not from ambition but from desperation.
Read Also: Photometry Explained: How We Measure Light the Way Humans See It
Max Planck’s Quantum Hypothesis
Origin of the Quantum Concept
Imagine being a scientist at the turn of the 20th century, staring at your equations and realizing—they just don’t work. That was Max Planck. Faced with the ultraviolet catastrophe, he did something revolutionary: he broke the rules of classical physics. He suggested that energy wasn’t continuous but came in small packets. That idea? It became known as energy quanta.
Planck didn’t set out to start a scientific revolution. He was just trying to fix a broken theory. But his workaround—treating energy as discrete units—was the spark that would light the fire of quantum physics.
Planck’s Constant and Quantized Oscillators
Here’s where it gets real. Planck proposed that each oscillator in an atom could only hold energy in specific amounts, based on frequency. To describe this, he introduced a new fundamental constant: Planck’s constant (h). This number, though small, has massive implications. It tells us how much energy a single quantum holds.
Formula E = hν Explained
This tiny formula—E = hν—changed everything. It means the energy (E) of a quantum is equal to Planck’s constant (h) times the frequency (ν) of radiation. No more infinite energy loops. No more physics nightmares. With this equation, quantum theory had its first real foundation. And Planck? He unknowingly opened the door to the weird and wonderful quantum world we explore today.
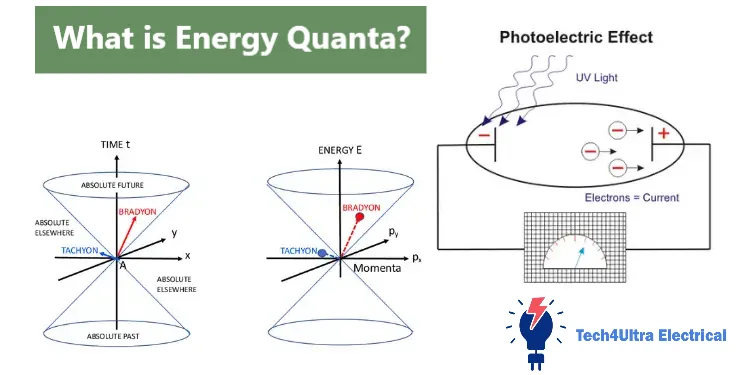
Einstein’s Photon Theory and the Photoelectric Effect
Explanation and Experimental Evidence
When I first heard that Einstein didn’t win the Nobel Prize for relativity but for explaining the photoelectric effect, I was honestly shocked. Turns out, this experiment was the ultimate test for quantum physics—and he nailed it.
Classical physics predicted that light, as a wave, should knock electrons off metal surfaces regardless of frequency—as long as the intensity was high enough. But in reality, low-frequency light, no matter how bright, did nothing. Meanwhile, high-frequency light, even if dim, kicked electrons out instantly. Weird, right?
Einstein solved the mystery by suggesting that light is made of tiny packets of energy—he called them photons. Each photon carries energy based on Planck’s formula, E = hν. If a photon has enough energy to overcome the metal’s threshold, it ejects an electron. If not, no ejection—no matter how many photons you throw at it.
Importance in Validating Quantization
This wasn’t just another physics puzzle. It was experimental proof that energy quanta were real. Light wasn’t just a wave—it behaved like particles too. The photoelectric effect became a cornerstone of quantum theory, proving that the microscopic world plays by different rules. Einstein didn’t just support Planck’s idea; he made it undeniable. And science? It would never look at light the same way again.
Wave–Particle Duality: Light and Matter
Dual Nature of Light (Wave and Particle)
Okay, so light acts like a particle. But wait—wasn’t it a wave too? That’s the crazy part. Light behaves like both. When I first learned this, it felt like science fiction. Turns out, it’s just quantum physics.
In some experiments, light creates interference patterns, which only waves can do. But in others, like the photoelectric effect, it acts like little particles—photons. This strange combo is called wave–particle duality. And it’s not just light. Electrons, and even larger particles, can do the same trick.
Electron Wave Experiments
The real shock came when scientists fired electrons—actual matter—at a double slit. Instead of just hitting the screen like bullets, they formed wave patterns. What?! Tiny solid particles acting like ripples on water?
This experiment proved that matter, like electrons, has wave-like properties too. It confirmed that energy quanta aren’t just for light. Quantum theory now applied to everything. Welcome to the quantum world—where reality is stranger than imagination.
Bohr’s Model and Quantized Atomic Orbits
Discrete Energy Levels in Atoms
Before Bohr, atoms were a mystery box. Electrons were thought to orbit randomly, and no one could explain why they didn’t just spiral into the nucleus. I remember staring at those textbook diagrams and thinking, “There’s got to be more to this.” And there was—thanks to quantum theory.
Bohr took Planck’s idea of energy quanta and gave it a home—in the atom. He proposed that electrons could only exist in specific orbits, each with its own energy level. No in-between. Electrons could jump between levels, but only by absorbing or emitting a specific quantum of energy.
Hydrogen Spectrum Explanation
This wasn’t just a neat theory. It explained something real: the hydrogen emission spectrum. When hydrogen gas is excited, it gives off light at very specific colors. Bohr showed that each line in that spectrum matched a jump between two energy levels in the atom.
This was a huge win for quantum physics. For the first time, we had a model that not only made sense but matched the data. The atom wasn’t chaotic—it was structured, ruled by discrete energy levels. And all of it pointed back to the same foundation: quantized energy.
Advancements in Quantum Mechanics
Schrödinger’s Wave Equation
If Bohr laid the groundwork, Schrödinger built the architecture. His wave equation blew my mind the first time I saw it. It treats particles like waves, describing how their energy and position evolve over time. No more imagining electrons as tiny balls—now they were blurry clouds of probability.
The genius of Schrödinger’s work was that it connected neatly with the concept of energy quanta. His equation could calculate the allowed energy levels in atoms—just like Bohr predicted—but with mathematical precision. Suddenly, quantum physics had a solid tool for predicting behavior on the smallest scales.
Heisenberg’s Uncertainty Principle
Then came Heisenberg to shake things up again. His uncertainty principle said we can’t know both the position and momentum of a particle exactly at the same time. At first, I thought, “That can’t be right—science is about measurements!” But he wasn’t talking about our tools. He meant it’s a fundamental limit of nature.
This principle wasn’t a flaw—it was the feature. It captured the essence of quantum theory: reality isn’t fixed until you look, and even then, it’s fuzzy.
Dirac, Pauli, and Quantum Field Theory
As if things weren’t wild enough, Dirac came along and merged quantum physics with Einstein’s relativity. That led to predicting antimatter—before it was even discovered. Pauli added the exclusion principle, explaining why matter doesn’t collapse in on itself. Then came quantum field theory, a framework where particles are just excitations in underlying fields. Complex? Absolutely. But it all traces back to that original idea of discrete energy levels—energy quanta—which started the quantum revolution.
Applications of Quantum Theory in Modern Technology
Semiconductors, Transistors, Lasers
When people say “quantum physics is hard and abstract,” I get it. But here’s what they don’t realize—it’s in your phone, your laptop, even the laser that scans groceries at the store. That’s the real magic of quantum theory. It’s not just equations on a chalkboard—it’s behind nearly every modern tech miracle.
Semiconductors, for instance, are built using quantum principles. Without understanding how electrons move in energy bands—yes, those same energy quanta—we wouldn’t have transistors. And without transistors? No computers, no smartphones, no internet.
Then there’s the laser—a perfect example of how controlling quantized energy can create powerful beams of light. It works by stimulating atoms to release photons in sync, using—you guessed it—quantum rules. You’ve seen lasers in everything from surgery to entertainment to fiber-optic communication. Quantum made that happen.
Quantum Computing and Cryptography
But the future? That’s where it gets wild. Quantum computing is set to redefine what computers can do. Instead of regular bits, it uses qubits—quantum bits that can represent multiple states at once thanks to superposition. The result? Potentially millions of calculations happening at once.
And let’s not forget quantum cryptography, which could make data breaches a thing of the past. It uses the principles of quantum theory—like entanglement and uncertainty—to create unbreakable encryption. If anyone tries to eavesdrop, the data changes. It’s like science fiction, only real.
So the next time someone says quantum is too complicated, just show them your phone. Quantum isn’t just changing science—it’s changing life.
Misconceptions and Challenges in Understanding Quantum Physics
Common Public Misunderstandings
Let’s be real—quantum physics has a reputation. People hear “quantum” and suddenly think time travel, alternate universes, or instant teleportation. I’ve had friends tell me quantum means “anything can happen,” which sounds cool, but isn’t how it works.
The biggest myth? That quantum theory is just guesswork or magic. In reality, it’s one of the most tested and accurate frameworks in science. Another common mix-up is thinking particles “choose” where to go. Truth is, they follow strict probability rules. Strange? Yes. Random chaos? Not really.
Philosophical Interpretations
Even scientists don’t fully agree on what quantum mechanics “means.” Some prefer the Copenhagen interpretation—where things aren’t real until measured. Others go for the Many-Worlds idea, suggesting every possibility actually happens somewhere else.
This debate isn’t about math—it’s about reality itself. That’s the challenge with energy quanta and everything quantum: it’s not just about what happens, but how we understand what’s happening. And while we’ve built incredible technologies with it, the deeper meaning is still up for grabs. That’s what makes quantum physics both frustrating and endlessly fascinating.
Watch Also: Understanding Space Charge: Effects, Laws, and Applications in Modern Electronics
Future of Quantum Physics and Emerging Trends
Quantum Entanglement
Here’s where quantum physics really starts to feel like sci-fi. Quantum entanglement is one of the weirdest things I’ve ever learned—two particles can become linked so perfectly that changing one instantly affects the other, even if they’re galaxies apart. Einstein called it “spooky action at a distance.” And honestly, that description fits.
But this isn’t just a cool thought experiment. Scientists have already used entanglement to transmit data across long distances without any physical link. That’s not just mind-blowing—it’s world-changing.
Quantum Communication and AI
These strange behaviors are shaping real-world innovations. Quantum communication could bring us completely secure messaging systems—data that’s literally impossible to hack, because observing it changes it. And then there’s quantum AI, where machine learning could be supercharged using quantum computers that process millions of possibilities at once.
All of this builds on one radical idea: that the universe runs on energy quanta, not smooth continuums. We’ve only just started unlocking what’s possible. With each step forward, quantum theory keeps proving that the future of technology—and maybe even reality itself—belongs to the quantum world.
Conclusion
From blackbody radiation to quantum computing, the concept of energy quanta has reshaped how we understand the universe. What started as a fix to a mathematical glitch sparked breakthroughs that gave us transistors, lasers, and a new vision of matter and light.
Quantum physics isn’t just a theory—it’s a pillar of modern science. It explains the structure of atoms, the behavior of particles, and the strange dual nature of light and matter. And it’s not done yet. As we move toward quantum communication, AI, and technologies we’ve yet to imagine, the principles of quantum theory remain more essential than ever.
So whether you’re a curious learner or a future innovator, understanding energy quanta isn’t just enlightening—it’s empowering.
FAQs
What is an energy quantum?
An energy quantum is the smallest possible unit of energy that a system can absorb or emit. Instead of being continuous, energy comes in these discrete packets. This concept is a core idea in quantum theory and helps explain how atoms and particles behave at microscopic scales.
What is the meaning of energy quantization?
Energy quantization means that energy levels are not continuous but exist in specific, fixed amounts. An electron in an atom, for example, can only occupy certain energy levels and must gain or lose a precise quantum of energy to move between them. This explains phenomena like atomic spectra.
What quanta means?
Quanta is the plural form of quantum. It refers to multiple packets or discrete units of something—usually energy. In quantum physics, quanta describe the tiny, indivisible amounts by which properties like energy and momentum can change.
What is quanta energy in chemistry?
In chemistry, quanta of energy often refer to the energy absorbed or emitted by electrons in atoms and molecules. When an atom absorbs energy, it does so in quantized amounts, causing electrons to jump to higher energy levels. This principle is essential in understanding chemical bonding, spectroscopy, and molecular interactions.