Contents
Have you ever wondered why different elements behave so differently, even though they’re made of the same basic particles? The answer lies in their electron configuration, also known as the electronic configuration of an atom. In this article on the Tech4Ultra Electrical website, you’ll discover how electrons are arranged within atoms—and how this arrangement impacts everything from chemical reactions to electrical conductivity. Whether you’re a student or just curious about chemistry, this guide will help make the concept crystal clear.
What is electron configuration?
I remember the first time I heard the term electron configuration in chemistry class. I nodded like I understood, but inside? Total confusion. It sounded technical, distant—like something only scientists in white coats needed to care about.
But here’s the thing: it’s actually super simple once you get it.
Electron configuration refers to the way electrons are arranged around the nucleus of an atom. Think of it like the seating chart at a concert—only instead of fans, you’ve got negatively charged electrons, and they can’t just sit anywhere they want. They follow specific “rules” based on energy levels and orbitals.
Each electron fills the lowest energy spot available, which makes the whole setup pretty predictable once you learn the order. You’ll often see configurations written like this: 1s² 2s² 2p⁶—don’t worry, we’ll break that down later.
Understanding this setup helped me realize that atoms aren’t random—they’re incredibly organized.
Why it matters in understanding atomic behavior
So, why should you care about the electronic configuration of an atom?
Because it’s basically the blueprint of how atoms interact with each other. Whether an element is reactive like sodium or noble and unbothered like helium, it all comes down to how its electrons are arranged.
- Elements with nearly full outer shells tend to gain electrons (hello, chlorine).
- Those with just one or two electrons in the outer shell love to give them away (like potassium).
- And noble gases? They’re full-up and want nothing to do with bonding.
Understanding electron configuration can explain why metals conduct electricity, why certain atoms form specific molecules, and even why elements in the same column of the periodic table behave similarly.
Honestly, once I “got it,” chemistry started making so much more sense.
Read Also: Plum Pudding Model Explained: Thomson’s Early Atomic Theory
The Atomic Model Evolution
From Dalton to Bohr to Quantum Model
When I first studied the history of atomic theory, I was amazed by how our understanding of the atom evolved over time. Back in the early 1800s, John Dalton pictured atoms as tiny, solid spheres—no internal structure, no fuss. It was a simple model, but it laid the groundwork.
Then came J.J. Thomson with his “plum pudding” model, introducing electrons as little negatively charged bits stuck in a positively charged soup. It sounded like dessert more than science—but hey, it was progress.
Ernest Rutherford shook things up with his gold foil experiment. He showed that atoms have a dense nucleus and that most of the atom is empty space. But the electrons? Still a mystery.
Enter Niels Bohr. His model placed electrons in specific orbits around the nucleus, like planets around the sun. Finally, electrons had structure and purpose. But it didn’t stop there.
The modern quantum model brought a whole new level of complexity. Electrons aren’t just in orbits—they exist in clouds of probability. We can’t pinpoint them exactly, but we know where they’re likely to be.
Highlight the role of electrons in atomic theory
Throughout each stage of atomic theory, electrons became more central. They explain chemical bonding, electrical conductivity, and why elements behave the way they do.
Understanding the electronic configuration of an atom is key to unlocking the secrets of the periodic table. From Dalton’s billiard balls to quantum clouds, the evolution of atomic models shows one thing clearly: electron configuration is at the heart of atomic science.
Quantum Numbers and Atomic Structure
Principal, Azimuthal, Magnetic, and Spin Quantum Numbers
Okay, I’ll admit it—the first time I saw the phrase “quantum numbers,” I thought I’d stumbled into advanced physics territory. But it turns out, they’re not that scary. In fact, these four numbers are the backbone of the electronic configuration of an atom.
First, there’s the principal quantum number (n). It tells you the main energy level, or shell, where an electron resides. Bigger number? Higher energy, further from the nucleus. Easy enough.
Then comes the azimuthal quantum number (l). This one defines the shape of the orbital—whether it’s spherical (s), dumbbell-shaped (p), or more complex (d and f). It’s like choosing the room an electron hangs out in.
The magnetic quantum number (ml) gives the orbital’s orientation in space. Multiple orbitals of the same shape can exist in different directions—this number points to one of them specifically.
Finally, there’s the spin quantum number (ms). Every electron has a spin, either +½ or -½. And since only two electrons can fit in each orbital, they must have opposite spins. This little detail plays a huge role in how atoms build up.
How they define orbitals and electron positions
So, why do these numbers matter? Because they work together to define the unique address of each electron in an atom. Just like a home address has a street, city, zip code, and apartment number—quantum numbers tell us where an electron lives, how it moves, and what shape its space has.
This is what gives structure to the electron configuration. Without quantum numbers, it would be impossible to predict where electrons go, or why certain configurations are more stable than others.
Energy Levels, Shells, and Subshells
Explanation of shells (K, L, M…) and sublevels (s, p, d, f)
One of the most satisfying “aha!” moments I had in chemistry was finally understanding how electron configuration ties into those mysterious letters—K, L, M, and the subshells like s, p, d, and f. At first, it felt like alphabet soup, but then it all clicked.
Each atom has energy levels, which are the “shells” labeled as K, L, M, N… or, more commonly in science-speak, n = 1, 2, 3, 4, and so on. These labels correspond directly to the principal quantum number (n). The higher the number, the farther the shell is from the nucleus and the more energy the electrons in it have.
Inside each shell are subshells—these are the s, p, d, and f orbitals. Think of them like rooms inside a building floor. The s subshell holds 2 electrons, p holds 6, d holds 10, and f can cram in 14. Each one has a distinct shape, and this layout helps determine how electrons fill up the atom.
Connection to principal quantum number
The principal quantum number doesn’t just tell you which shell an electron is in—it also limits which subshells are available. For example, if n = 2 (the L shell), then only the 2s and 2p subshells exist. No 2d or 2f allowed.
This connection is crucial in building an accurate electronic configuration of an atom. Understanding these energy levels helps you predict chemical properties, like reactivity and bonding behavior. Once you visualize the shells and subshells as a layered structure, the whole atom becomes way less intimidating.
Aufbau Principle and Orbital Filling Rules
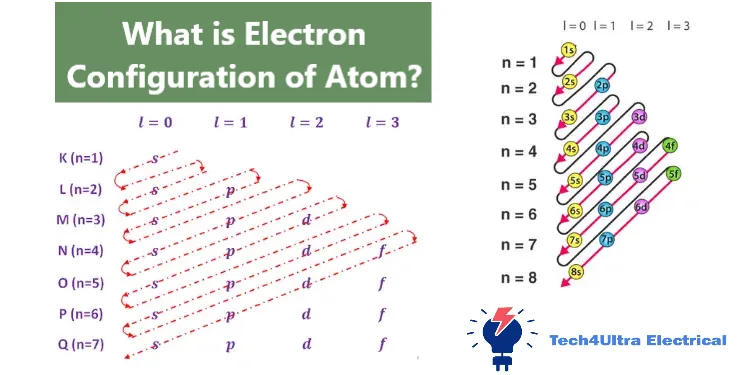
The n+ℓ rule and Madelung diagram
If I had a dollar for every time I messed up the order of orbital filling, I’d have enough to buy a periodic table poster for every wall in my room. Understanding how electron configuration builds up isn’t always intuitive at first—but the Aufbau principle really helps make sense of it.
“Aufbau” is a German word that means “building up,” and that’s exactly what this principle is about: electrons fill the lowest available energy levels first. You don’t just toss electrons into orbitals randomly; there’s a very specific order, and it’s guided by the n+ℓ rule.
Here’s how it works:
- n = principal quantum number (energy level)
- ℓ = azimuthal quantum number (subshell type)
Add those together, and you get a priority number. Orbitals with the lowest n+ℓ value get filled first. If two orbitals have the same total, the one with the lower n value goes first. This is the logic behind the Madelung diagram, which visually maps out the order of orbital filling.
Order of orbital filling with visual examples
Even though I can’t draw here, let’s walk through a simplified version of the order:
- 1s
- 2s
- 2p
- 3s
- 3p
- 4s
- 3d
- 4p
- 5s
- 4d
- 5p
- 6s
- 4f
- 5d
- 6p
- 7s
This list looks long, but with a bit of practice, it becomes second nature. I used to write it on the edge of my notebook during quizzes until it stuck in my head.
The beauty of the Aufbau principle is that it gives structure and predictability to the electronic configuration of an atom. Instead of guessing, you follow the rules, and the electrons fall into place like puzzle pieces.
How to Write Electron Configurations
Full configuration notation
Writing the full electron configuration of an atom used to feel like decoding a secret message. But once I got the hang of the structure, it started to feel more like filling seats in a concert hall—orderly and logical.
Start with hydrogen: it has one electron, so its configuration is simply 1s¹. Helium gets a second electron, so it’s 1s². From there, the process builds:
Take oxygen, for example. With eight electrons, its full electronic configuration is:
1s² 2s² 2p⁴
The numbers tell you the energy level (1, 2), the letter shows the subshell (s, p), and the superscript shows how many electrons are in that subshell. Just follow the filling order (from the Aufbau principle), and you’re good.
Noble gas shorthand method
Here’s where things get convenient. For larger atoms, writing the full configuration every time is exhausting. Instead, you can use the noble gas shorthand.
Let’s say you’re dealing with calcium (20 electrons). Instead of writing all the way from 1s² to 4s², you can replace the first 18 electrons with argon’s symbol:
[Ar] 4s²
This shorthand saves time and space, and still gives a clear picture of where the remaining electrons go.
Tips for avoiding common mistakes
- Don’t forget the filling order—3d comes after 4s, not before.
- Double-check subshell capacities: s (2), p (6), d (10), f (14).
- Use periodic table blocks (s, p, d, f) as a map—it’s a lifesaver.
- Always verify electron counts match the atomic number.
Getting fluent in writing electron configuration just takes a few reps. I used to practice by writing out configurations for random elements—now it’s second nature. Trust me, the more you do it, the easier it gets.
Exceptions in Electron Configuration
Chromium, Copper, and other anomalies explained
Just when I thought I had electron configuration all figured out, along came Chromium and Copper to mess with everything. These two don’t follow the normal Aufbau order, and honestly, that used to drive me nuts.
Let’s look at Chromium first. Its atomic number is 24, so we’d expect the configuration to be:
[Ar] 4s² 3d⁴
But surprise—it’s actually:
[Ar] 4s¹ 3d⁵
Why? Because half-filled d-orbitals (like 3d⁵) are more stable. Chromium “borrows” one electron from 4s to achieve this stability.
Same with Copper (atomic number 29). Expected:
[Ar] 4s² 3d⁹
Actual:
[Ar] 4s¹ 3d¹⁰
Here, a full d-orbital (3d¹⁰) is more stable than the expected layout. So again, it shuffles an electron to get to a lower-energy configuration.
Why deviations occur from expected order
These exceptions aren’t random—they’re about electronic configuration stability. Half-filled and fully filled subshells offer extra stability due to symmetrical electron distribution and minimized electron repulsion.
So when you see these exceptions, remember: atoms are trying to reach the most stable arrangement possible, even if it means breaking a few “rules.” Once I understood this logic, the anomalies stopped feeling like mistakes—and started making perfect sense.
Examples for Common Elements
Detailed configurations for Hydrogen, Carbon, Sulfur, Calcium, and Iron
Now that we’ve covered the theory, let’s apply it to some real elements. Practicing actual electron configuration examples helped me remember the rules more than anything else. Let’s break down a few:
- Hydrogen (1 electron):
1s¹ - Carbon (6 electrons):
1s² 2s² 2p² - Sulfur (16 electrons):
1s² 2s² 2p⁶ 3s² 3p⁴ - Calcium (20 electrons):
[Ar] 4s² - Iron (26 electrons):
[Ar] 4s² 3d⁶
Each of these shows how electrons fill the shells and subshells in a predictable pattern—until you reach transition metals like Iron, where the d-block brings some complexity.
Practice problems with answers
Try figuring out these yourself before checking the answers:
- What is the electron configuration for Neon (10 electrons)?
Answer:1s² 2s² 2p⁶ - Write the noble gas shorthand for Zinc (30 electrons).
Answer:[Ar] 4s² 3d¹⁰ - How many electrons are in the 3p orbital of Chlorine (17 electrons)?
Answer: 5 electrons
Working through these examples makes electronic configuration way more intuitive. The more elements you practice, the more natural it becomes to predict how electrons arrange themselves.
The Octet Rule and Chemical Stability
Why atoms seek full valence shells
Ever wonder why some elements are so reactive while others like neon just chill? It all comes down to the octet rule—the idea that atoms are most stable when they have eight electrons in their outer shell. It’s chemistry’s version of “mission accomplished.”
When I first learned this, it clicked: atoms aren’t just hanging around randomly—they’re on a mission to achieve a full valence shell. This is especially true for main-group elements. Oxygen wants two electrons to reach eight, so it reacts easily. Sodium, with one lonely outer electron, would rather donate it to feel complete.
Why eight? Because that’s what makes noble gases like argon and neon so unreactive. Their electronic configuration is already perfect—they’ve hit the jackpot of atomic stability.
Link to noble gas configuration and bonding
This craving for stability drives chemical bonding. Atoms gain, lose, or share electrons just to imitate the electron configuration of the nearest noble gas. Whether it’s ionic bonds (electron transfer) or covalent bonds (electron sharing), the goal is always the same: a stable octet.
Once I understood the octet rule, suddenly chemical reactions felt less chaotic and more purposeful. It’s all about reaching that calm, stable energy state. Just like people, atoms want to feel balanced.
Watch Also: Planar and Non-Planar Graphs Explained with Circuit Examples
Interactive Tools and Learning Aids
Recommended online electron configuration calculators and periodic tables
Let’s be honest—memorizing every electron configuration manually can be overwhelming. What saved me? Interactive tools. There are some fantastic online calculators that give you instant electronic configuration of an atom just by entering the element’s name or number.
My upgraded go‑to resources include:
- ChemicalAid Electron Configuration Calculator – Instantly generates full and shorthand electron configurations for any element.
- Omni Calculator – Electron Configuration Tool – Includes explanations of ground‑state fill order, Hund’s rule, and valence electrons, updated ~2.5 years ago.
- dCode Electron Configuration Converter – Lets you input atomic numbers or full configurations and it parses them; practical for problem-solving.
- ExploreLearning Gizmos: Electron Configuration Virtual Lab – Interactive animation tool linked to classroom learning, refreshed ~5 months ago.
- Electron Config Pro (Android App) – Offers orbital animations, quizzes (~80 questions), and covers multiple oxidation states; actively updated.
Visual animations to reinforce learning
If you’re a visual learner like me, animations can change the game. Sites like Khan Academy and YouTube channels like CrashCourse break down the orbital filling process with graphics that actually stick in your mind.
These tools made electron configuration feel real, not just theoretical. Trust me—once you watch those electrons zoom into orbitals, the whole concept clicks.
Conclusion
Recap of main points
If you’ve made it this far—congrats! You’ve covered a lot. From understanding what electron configuration means to decoding quantum numbers, energy levels, and orbital filling rules, each part builds your ability to read and write the electronic configuration of an atom with confidence.
We looked at real examples like Carbon and Iron, tackled exceptions like Chromium, and learned tricks like noble gas shorthand. We also explored interactive tools that make studying easier and more fun.
Importance of mastering electron configuration in chemistry
Why does this all matter? Because electron configuration is the language behind chemical behavior. It explains reactivity, bonding, stability, and even the periodic table’s shape. Once you understand how electrons are arranged, chemistry stops feeling random—and starts making sense.
So whether you’re prepping for a test, diving into advanced studies, or just curious, mastering this topic is a major key to unlocking deeper understanding in chemistry.
FAQs
How do you understand electron configuration?
Start by breaking it down step by step. The electron configuration of an atom shows how its electrons are arranged across different energy levels and orbitals. Learn the rules like the Aufbau principle, Pauli exclusion, and Hund’s rule—they guide how electrons fill the orbitals. With practice, these patterns start to make sense, and writing configurations becomes second nature.
How to find the electronic configuration of an atom?
First, determine the atom’s atomic number—it tells you the total number of electrons. Then follow the filling order: 1s → 2s → 2p → 3s → 3p → 4s → 3d.... Use the noble gas shorthand for larger atoms by substituting core electrons with a noble gas in brackets. Online calculators can also help verify your work if you’re just starting out.
What is the meaning of 1s, 2s, 2p, 3s, 3p?
These are part of the electronic configuration notation. The number (1, 2, 3…) is the energy level (or shell), the letter (s, p, d, f) is the subshell or orbital type, and the superscript (like the “²” in 1s²) tells you how many electrons are in that orbital. For example, 2p⁶ means six electrons are in the second energy level’s p orbital.
How to learn electronic configuration easily?
Use visual tools like periodic tables and orbital diagrams. Write out configurations for common elements. Practice using the Madelung diagram and n+ℓ rule to understand filling order. And don’t forget interactive platforms like Ptable or Electron Config Pro—they turn theory into something visual and intuitive. Repetition and breaking it into chunks worked wonders for me!

4 thoughts on “Planar and Non-Planar Graphs Explained with Circuit Examples”