Contents
Have you ever wondered how something can be both a particle and a wave at the same time? It sounds like a paradox, but that’s exactly what the wave particle duality principle in quantum physics reveals. In this article on the Tech4Ultra Electrical website, you’ll discover how this mind-bending concept reshaped our understanding of light, matter, and reality itself—explained in a simple and engaging way. If you’ve ever been curious about how the universe really works beneath the surface, you’re in the right place.
The wave particle duality is one of the most fascinating and foundational principles in quantum mechanics. It refers to the concept that every particle or quantum entity, such as an electron or photon, exhibits both wave-like and particle-like properties depending on how it is observed. This dual behavior challenges classical physics, which traditionally categorized matter as either a wave or a particle, never both.
Understanding the wave particle duality principle is crucial because it helps explain a wide range of phenomena that cannot be interpreted by classical theories. For example, light can behave like a wave when it diffracts or interferes, and like a particle when it transfers energy in discrete packets called photons.
This duality was first brought to light in experiments like the double-slit experiment, where particles such as electrons created an interference pattern—something previously thought to be possible only with waves. The implications of this discovery are profound, laying the groundwork for technologies like electron microscopes and quantum computing.
In short, wave particle duality redefined the very nature of reality at the microscopic level and continues to be a cornerstone of modern quantum theory.
Historical Background and Classical Theories
Long before the development of quantum mechanics, scientists struggled to understand the true nature of light. In the 17th century, Sir Isaac Newton proposed the corpuscular theory, suggesting that light is made up of small particles, or “corpuscles.” According to Newton, these particles traveled in straight lines and explained phenomena like reflection and refraction quite effectively. His theory aligned well with the ideas of motion and force in classical mechanics, making it widely accepted at the time.
However, around the same period, Dutch physicist Christiaan Huygens presented a different view—what we now call the wave theory of light. Huygens argued that light behaves like a wave, propagating through space much like ripples on water. This theory explained interference and diffraction patterns, which Newton’s model could not account for. But the wave theory also had its weaknesses, especially in explaining why light seems to travel in straight lines and how it interacts with individual particles of matter.
By the 19th century, experiments such as Thomas Young’s double-slit experiment strongly supported the wave theory by demonstrating clear interference patterns. Yet, when scientists later observed phenomena like the photoelectric effect, it became evident that light also behaved like a stream of particles—reviving Newton’s earlier ideas in a new light.
These classical theories laid the groundwork, but neither alone could fully describe the behavior of light. This clash of interpretations eventually led to the emergence of the wave particle duality concept in the early 20th century, revolutionizing how we view energy and matter.
Read Also: Energy Quanta: The Foundation of Quantum Theory
Quantum Breakthroughs in Light Duality
The true turning point in understanding the wave particle duality came with several groundbreaking discoveries in the early 20th century. One of the most influential was Albert Einstein’s explanation of the photoelectric effect. Classical wave theory could not explain why light below a certain frequency failed to eject electrons from a metal surface, regardless of its intensity. In 1905, Einstein proposed that light consists of discrete packets of energy—photons—each carrying energy proportional to its frequency. This idea built on Max Planck’s relation E = hf, where E is the energy of the photon, h is Planck’s constant, and f is the frequency of light.
This quantum leap solved the puzzle: if a single photon didn’t have enough energy (low frequency), no electrons would be emitted, no matter how many photons arrived. But if just one photon had enough energy, it could instantly eject an electron. This confirmed the particle nature of light and earned Einstein the Nobel Prize in Physics in 1921.
Another significant experiment was Arthur Compton’s study of X-ray scattering in 1923, known as Compton scattering. When X-rays were directed at electrons, the scattered rays had lower energy, and the electrons recoiled like particles struck by billiard balls. This confirmed that photons not only have energy but also momentum, reinforcing the concept of light as a quantum particle.
These discoveries revealed that light isn’t purely a wave or a particle—it is both, depending on how it’s observed. They forced the scientific community to abandon classical models and embrace the more complex but accurate framework of quantum mechanics. The wave particle duality principle became not just a theoretical curiosity, but a cornerstone of modern physics, shaping everything from semiconductors to lasers and beyond.
wave particle duality in Matter
The wave particle duality wasn’t just limited to light. In 1924, French physicist Louis de Broglie took a bold step and proposed that particles of matter, like electrons, also exhibit wave-like behavior. He suggested that any moving particle has an associated wavelength, given by the formula λ = h/p, where λ is the wavelength, h is Planck’s constant, and p is the momentum of the particle. This revolutionary idea extended the wave particle duality principle to all matter, not just photons.
At first, de Broglie’s hypothesis was purely theoretical. But in 1927, the Davisson–Germer experiment provided solid evidence. While studying electron scattering off a nickel crystal, Clinton Davisson and Lester Germer observed an interference pattern—a clear sign of wave behavior. Their experiment, which unintentionally aligned perfectly with crystal lattice spacing, confirmed that electrons can indeed diffract like waves. This finding shocked many and validated de Broglie’s concept of matter waves.
Around the same time, G.P. Thomson, son of J.J. Thomson (the discoverer of the electron), conducted a different but equally important experiment. He fired high-energy electrons through thin metal foils and observed diffraction rings, just like those seen in X-ray diffraction. Again, electrons displayed wave-like interference, offering another powerful confirmation of their dual nature.
These experiments marked a pivotal moment in physics. They showed that not only does light exhibit wave particle duality, but so does matter. The concept of matter waves became central to quantum mechanics, leading to the development of quantum wave equations—most notably Schrödinger’s wave equation, which treats particles as wave functions.
The implication is profound: everything in the universe, from tiny electrons to larger particles, possesses both wave and particle properties. The wave particle duality is not just a quirk of photons but a fundamental trait of all quantum objects, forever changing how we interpret the physical world.
The Double-Slit Experiment Explained
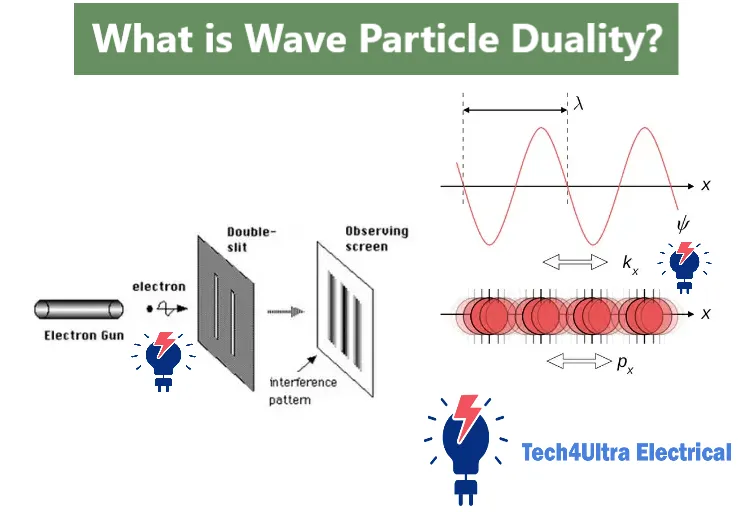
If there’s one experiment that truly brings the wave particle duality to life, it’s the famous double-slit experiment. Originally conducted with light by Thomas Young in 1801, it showed that light passing through two slits creates an interference pattern on a screen—something only waves could do. This was well understood from a classical perspective. But things took a dramatic turn when the same experiment was performed with individual particles, like electrons.
In the quantum version, electrons were fired one by one at a barrier with two slits, and surprisingly, over time they built up an interference pattern on the screen behind—just like waves. The twist? Each electron was detected as a single point-like particle, but collectively they behaved like a wave interfering with itself. This made no sense in classical physics, where particles should just form two clusters behind the slits.
The mystery deepens when an observer is introduced. If a detector is placed at the slits to observe which slit the electron goes through, the interference pattern disappears, and the electrons behave like particles again. This phenomenon—where the act of observation collapses the wave function—is one of the most bizarre aspects of quantum mechanics. It suggests that particles exist in a state of probability, or superposition, until they are observed.
This collapse of the wave function shows how measurement itself changes the outcome. The wave particle duality principle here is not just a theoretical idea—it’s an observable, measurable effect that hinges on whether we choose to watch. The double-slit experiment remains a cornerstone in demonstrating how deeply different quantum mechanics is from our everyday intuition and why it continues to challenge even the brightest minds in science.
Quantum Principles Related to Duality
To fully grasp the implications of the wave particle duality, we need to explore some key quantum principles that support it. One of the most important is Niels Bohr’s complementarity principle. This concept states that wave and particle aspects of a quantum object are complementary—meaning you can observe one or the other, but never both at the same time. The behavior you observe depends entirely on how you set up the experiment. In other words, nature doesn’t “choose” until you measure.
Another fundamental principle is Heisenberg’s uncertainty principle. It states that you can’t simultaneously know both the exact position and the exact momentum of a particle. The more precisely you know one, the less precisely you can know the other. This isn’t a limitation of instruments—it’s built into the nature of quantum systems. This uncertainty directly ties into the wave particle duality principle, because particles with wave-like properties can’t have well-defined locations and velocities at the same time.
Finally, the Schrödinger equation brings it all together. This famous equation describes how a quantum system evolves over time. Instead of giving the exact location of a particle, it produces a wave function—a mathematical function that contains all the probabilities of where the particle might be. This wave function is the core representation of a quantum object’s dual nature.
Together, these principles explain why we can’t rely on classical logic in the quantum world. They form the foundation for interpreting the strange but beautifully consistent behavior revealed by the wave particle duality.
Watch Also: Schrödinger Wave Equation Explained: From Basics to Derivation
Modern Applications of wave particle duality
The Wave–particle duality isn’t just a mind-bending concept—it’s the engine behind some of the most advanced technologies of our time. One powerful example is the electron microscope. Unlike traditional microscopes that use light, electron microscopes use electron beams. Thanks to their wave-like behavior, electrons can have much shorter wavelengths than visible light, allowing scientists to view structures at the atomic scale. Without embracing the wave nature of matter, this breakthrough would’ve been impossible.
Another cutting-edge field built on the wave particle duality principle is quantum computing. Quantum bits, or qubits, rely on the superposition of states—a concept rooted in wave functions. Because quantum particles can exist in multiple states simultaneously, quantum computers can solve problems exponentially faster than classical ones. The very fabric of their logic depends on wave-like behaviors and the interference patterns they produce.
The Mach-Zehnder interferometer is another brilliant application. It’s a device that splits a particle’s path into two, allowing them to interfere with each other—just like in the double-slit experiment. This technique is crucial in testing quantum theories and building precision measurement tools in labs and satellites. It also plays a role in developing ultra-sensitive sensors and quantum communication systems.
These aren’t just theoretical curiosities. Technologies based on the wave particle duality are shaping everything from medical diagnostics to secure internet protocols. Our growing mastery over wave-particle phenomena is unlocking a future where science fiction becomes science fact. Whether it’s looking deep into a cell or encrypting data with quantum keys, this principle remains a pillar of innovation in the 21st century.
Conclusion
The wave particle duality has transformed our understanding of the physical world. From light acting as both a wave and a stream of particles, to electrons displaying interference patterns, this principle shattered the classical view of physics. Thanks to groundbreaking work from scientists like Einstein, de Broglie, and others, we now understand that particles and waves are two sides of the same quantum coin.
Core concepts like the photoelectric effect, electron diffraction, and Heisenberg’s uncertainty principle all stem from the wave particle duality principle. These insights not only explain bizarre quantum behavior but also fuel innovations like quantum computing, advanced imaging techniques, and quantum communication.
Looking ahead, the duality continues to play a central role in quantum research. From exploring the nature of consciousness to investigating quantum gravity, physicists are still grappling with the implications of this principle. It’s a vivid reminder that the universe doesn’t always follow human intuition—but with each discovery, we get one step closer to unlocking its deepest secrets.
FAQs
Is wave–particle duality proven?
Yes, the wave particle duality is supported by extensive experimental evidence. From the double-slit experiment to the photoelectric effect and electron diffraction, countless studies confirm that particles like photons and electrons exhibit both wave-like and particle-like behavior depending on how they’re measured. It’s one of the most well-established principles in quantum mechanics.
Can particles behave like waves?
Absolutely. According to the wave particle duality principle, every particle has an associated wavelength, as shown by de Broglie’s equation (λ = h/p). This wave behavior has been directly observed in experiments like the Davisson–Germer and G.P. Thomson experiments. Even large molecules, like buckyballs (C60), have shown wave interference under the right conditions!
Why does observation affect results?
In quantum mechanics, observation is not passive. Measuring a system forces it to “choose” a definite state—a process known as wave function collapse. This is why, in the double-slit experiment, interference disappears when we try to observe which slit a particle goes through. The act of observation alters the outcome, highlighting the core weirdness and beauty of the wave particle duality.
What is the wave-particle duality principle?
The wave particle duality principle is a fundamental concept in quantum mechanics stating that every quantum entity—such as a photon, electron, or even atom—exhibits both wave-like and particle-like properties. Depending on the type of experiment performed, these entities may behave like discrete particles or continuous waves, but never both simultaneously. It’s a cornerstone of our modern understanding of quantum physics.
What is the duality principle of radiation?
The duality principle of radiation refers to the idea that electromagnetic radiation, like light or X-rays, behaves both as a stream of particles (photons) and as a wave. This dual nature was confirmed through phenomena such as the photoelectric effect (particle behavior) and interference/diffraction patterns (wave behavior). The wave particle duality helps explain how light can be absorbed and emitted in discrete amounts while also propagating through space as a wave.
What is the uncertainty principle of wave-particle duality?
Heisenberg’s uncertainty principle complements the wave particle duality principle by stating that it’s impossible to simultaneously know a particle’s exact position and momentum. The more precisely you know one, the less you know the other. This uncertainty arises naturally because quantum objects behave like waves—waves don’t have a single defined position, making precise measurement inherently limited.
What is the wave-particle duality concept by Albert Einstein?
Albert Einstein’s contribution to the wave particle duality came through his work on the photoelectric effect. He proposed that light consists of particles called photons, each carrying energy based on its frequency. This explained why only light above a certain frequency could eject electrons from metal surfaces. Einstein’s insight confirmed the particle aspect of light and laid the foundation for quantum theory, earning him the Nobel Prize in 1921.