Contents
Did you know that even the darkest object in the universe emits radiation? This phenomenon, known as black body radiation, is not just theoretical—it’s fundamental to understanding how all objects emit heat and light. In this article on the Tech4Ultra Electrical website, we’ll break down essential concepts like Planck’s law, the Stefan–Boltzmann law, and Wien’s displacement law. Whether you’re a student, a science enthusiast, or just curious, you’ll walk away with a clear understanding of how these laws reveal the hidden energy all around us.
A black body is a theoretical object that absorbs all incident electromagnetic radiation, regardless of frequency or angle. Unlike real-world materials, a perfect black body reflects nothing and emits radiation purely based on its temperature. This emitted radiation is known as black body radiation, and it follows predictable physical laws.
Understanding black body radiation is a cornerstone in both physics and engineering. Why? Because it laid the foundation for quantum mechanics. When classical physics couldn’t explain the spectrum of radiation from a black body, Planck’s law emerged as a groundbreaking solution, marking the birth of modern physics. This law explains how energy is emitted in discrete packets, or quanta, rather than in a continuous wave.
Beyond theoretical importance, the concept plays a critical role in practical technologies. In space science, for instance, scientists use black body models to estimate the temperature of stars and planets. Similarly, thermal cameras rely on these principles to detect infrared radiation and visualize heat. Even climate models and remote sensing satellites depend on black body assumptions to interpret thermal emissions from Earth.
In essence, black body radiation isn’t just a scientific curiosity—it’s a key that unlocks insights into the natural and technological world.
What is a Perfect Black Body?
A perfect black body is an idealized physical object that absorbs all incoming radiation, regardless of wavelength or angle of incidence. It neither reflects nor transmits any part of the electromagnetic energy that falls on it. This makes it the most efficient emitter of radiation possible in physics.
The defining characteristic of a black body is its emissivity value. Emissivity is a measure of how effectively a surface emits thermal radiation compared to a perfect emitter. For a perfect black body, emissivity equals 1. This means it emits the maximum amount of radiation possible at a given temperature according to Planck’s law.
In contrast, a gray body has an emissivity less than 1 and emits less radiation than a black body at the same temperature. Real-world materials, like metals or ceramics, fall somewhere in between, with emissivities varying based on surface finish, temperature, and wavelength.
While a true black body does not exist in nature, certain materials and cavities can closely approximate its behavior. These approximations are critical in laboratories and engineering applications for calibrating instruments and studying thermal radiation.
Read Also: Photometry Explained: How We Measure Light the Way Humans See It
Understanding Thermal Radiation
Thermal radiation is the energy emitted by a body due to its temperature. As the temperature of an object increases, it emits more radiation, and the nature of this radiation changes. Every object with a temperature above absolute zero emits electromagnetic radiation, and the intensity and wavelength of that radiation depend on its temperature.
As temperature rises, the emitted radiation becomes more intense and shifts to shorter wavelengths. This is described by Wien’s displacement law, which explains why hot objects glow—like when metal turns red, then white as it gets hotter. Cooler objects emit mostly in the infrared (IR) part of the spectrum, which is invisible to the human eye but detectable using thermal cameras.
The electromagnetic spectrum includes a range of radiation types, from radio waves to gamma rays. Thermal radiation mainly spans the infrared and visible regions. Objects at room temperature emit radiation primarily in the infrared range, while objects at higher temperatures (like the sun) emit significant energy in the visible range.
Understanding this relationship between temperature and emitted radiation is essential in applications like climate science, astronomy, and engineering. It helps us analyze how objects radiate heat and how sensors interpret this energy in real-world systems.
The Three Key Laws of Black Body Radiation
a. Planck’s Law
Planck’s law describes the spectral distribution of black body radiation. It tells us how much energy a black body emits at each wavelength, depending on its temperature. The law is represented mathematically as:
B(λ, T) = (2hc² / λ⁵) / (e^(hc / λkT) - 1)
Where:
- B(λ, T) is the spectral radiance
- λ is the wavelength
- T is the absolute temperature in kelvins
- h is Planck’s constant
- c is the speed of light
- k is Boltzmann’s constant
This formula was revolutionary because classical physics couldn’t explain the observed radiation spectrum, especially at shorter wavelengths—a problem known as the “ultraviolet catastrophe.” Planck’s law resolved this by introducing the idea of energy quantization.
The spectral radiance curves generated from this law show that as temperature increases, the peak of the radiation shifts to shorter wavelengths and the total emitted energy increases. These curves are essential in astrophysics and thermal imaging.
b. Wien’s Displacement Law
Wien’s displacement law shows the relationship between the temperature of a black body and the wavelength at which it emits most strongly. The law is given by:
λ_max = b / T
Where:
- λ_max is the peak wavelength in meters
- T is the temperature in kelvins
- b is Wien’s constant (approximately 2.898 × 10⁻³ m·K)
This means that hotter objects emit radiation at shorter wavelengths. For instance, the sun emits mostly in the visible range because of its high temperature, while cooler objects emit primarily in the infrared.
In practical terms, Wien’s law helps in determining the temperature of stars and planets by analyzing their radiation spectra. It’s also used in thermal sensors and cameras to measure temperature based on emitted light.
c. Stefan–Boltzmann Law
The Stefan–Boltzmann law quantifies the total energy emitted per unit surface area of a black body across all wavelengths. The law is expressed as:
P = σT⁴
Where:
- P is the power emitted per unit area
- T is the absolute temperature in kelvins
- σ is the Stefan–Boltzmann constant (5.67 × 10⁻⁸ W/m²·K⁴)
This law shows that the total radiated energy increases rapidly with temperature. Doubling the temperature of an object results in 16 times more energy being emitted.
The Stefan–Boltzmann law is widely used in climate science, engineering, and astronomy. For example, it’s essential in estimating the luminosity of stars and understanding how Earth absorbs and emits heat in energy balance models.
Supporting Theories and Historical Development
Before Planck’s law revolutionized physics, scientists struggled to understand the nature of black body radiation. One key foundation came from Kirchhoff’s law of thermal radiation, which states that for a body in thermal equilibrium, the emissivity equals the absorptivity at every wavelength. This principle reinforced the idea of a perfect black body as an ideal emitter and absorber.
However, classical physics ran into a major problem—known as the ultraviolet catastrophe. According to Rayleigh–Jeans law, the energy emitted by a black body should increase infinitely at shorter wavelengths, which obviously contradicts experimental observations. This failure signaled a deeper issue in the classical approach to thermal radiation.
Enter Max Planck. In 1900, Planck introduced a radical idea: energy is emitted or absorbed in discrete units called quanta. By applying this concept, he derived what we now call Planck’s law, which matched observed spectral data perfectly. This marked the birth of quantum theory, laying the groundwork for a new era in physics.
Planck’s contribution went beyond explaining radiation—it reshaped how scientists view energy, light, and matter at the atomic level. His work eventually led to major developments in quantum mechanics, affecting everything from atomic models to modern electronics.
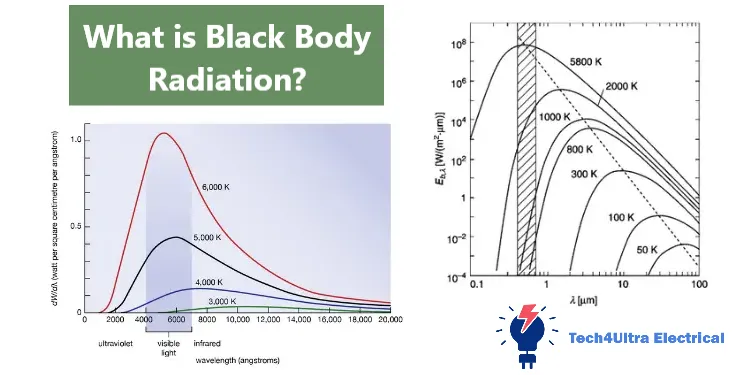
Graphical Analysis: Spectra at Different Temperatures
One of the most visually compelling ways to understand black body radiation is through spectral graphs. These graphs show how the intensity of radiation varies with wavelength for different temperatures. According to Planck’s law, as a black body heats up, two major changes occur in its radiation curve.
First, the peak of the curve shifts to the left—toward shorter wavelengths. This behavior is described by Wien’s displacement law. For example, a black body at room temperature emits primarily in the infrared, which is invisible to the naked eye. As the temperature increases, the peak moves into the visible light range, which is why heated objects glow red, orange, or even white-hot.
Second, the overall area under the curve increases dramatically, as explained by the Stefan–Boltzmann law. This area represents the total power emitted. Since radiation grows with the fourth power of temperature (T⁴), a small temperature increase leads to a significant jump in emitted energy.
When you examine a series of spectral curves, you’ll notice each one rising more sharply and peaking at shorter wavelengths as temperature increases. This helps scientists analyze stars, planets, and even industrial heat sources by matching real-world data to ideal black body behavior.
In summary, these graphs aren’t just theoretical—they are tools that reveal temperature, composition, and radiation efficiency through clear, measurable patterns.
Applications of Black Body Radiation
The concept of black body radiation is not just a theoretical construct; it plays a critical role in multiple scientific and engineering applications. One of the most well-known uses is in astrophysics, where stars are often modeled as black bodies. By analyzing the spectrum of a star’s emitted radiation and applying Wien’s displacement law, astronomers can determine the star’s surface temperature. For instance, our Sun closely follows the behavior predicted by Planck’s law, emitting most of its energy in the visible range.
Another vital application is in infrared thermography. This technology uses sensors to detect infrared radiation emitted by objects, allowing for temperature measurements without physical contact. It’s used in everything from diagnosing electrical faults and insulation issues in buildings, to medical imaging and night vision equipment. These devices rely on the assumptions of black body radiation to accurately interpret thermal emissions.
In climate science, black body models help simulate how the Earth and atmosphere absorb and emit radiation. Satellite sensors designed to observe Earth’s energy balance use the principles of Stefan–Boltzmann law to estimate surface temperatures and track changes in heat absorption and emission. These models are crucial for understanding global warming, cloud behavior, and energy exchanges between the Earth and space.
On a cosmic scale, one of the most groundbreaking confirmations of black body radiation came from the discovery of the Cosmic Microwave Background (CMB). This faint glow, a remnant from the early universe, matches the spectrum of a nearly perfect black body at about 2.7 K. The CMB provides a snapshot of the universe just 380,000 years after the Big Bang and serves as a cornerstone in cosmology.
From measuring star temperatures to scanning thermal leaks in homes and interpreting the origins of the universe, the applications of black body radiation span across disciplines—proving that a deep understanding of this phenomenon is both practical and profound.
Watch Also: Energy Quanta: The Foundation of Quantum Theory
Black Body vs Real Bodies
While a perfect black body is a theoretical construct, many real-world objects behave similarly under certain conditions. For instance, stars, including our Sun, act as near-perfect black bodies in terms of their emitted radiation. Other examples include black paint, which absorbs most incident light, and materials like graphite, which have high emissivity.
A classic physical approximation of a black body is a cavity with a small hole. When radiation enters the hole, it undergoes multiple reflections within the cavity, getting absorbed with minimal reflection. This setup is used in laboratory black body sources for calibrating infrared instruments and thermometers.
In contrast, a gray body emits a fixed fraction of the radiation a black body would emit at the same temperature. Its emissivity is less than 1 and remains constant across wavelengths. Most real materials are gray bodies with wavelength-dependent properties, meaning they don’t emit uniformly across the spectrum.
Understanding the difference between black bodies and real objects helps improve the accuracy of thermal modeling and enhances the design of sensors, optics, and thermal imaging devices.
Conclusion
Black body radiation serves as a cornerstone in both thermal physics and quantum mechanics. Through Planck’s law, Wien’s displacement law, and the Stefan–Boltzmann law, scientists can describe how objects emit radiation based on temperature. These principles not only resolved classical problems like the ultraviolet catastrophe but also launched the quantum revolution.
From analyzing the heat of stars in astrophysics to calibrating thermal cameras and developing accurate climate models, the applications of black body theory are vast and impactful. Even the cosmic microwave background—a remnant of the Big Bang—confirms the predictive power of these laws.
Understanding black body radiation is more than an academic exercise; it’s a key to decoding how energy moves and transforms across the universe. Whether you’re studying physics or solving real-world problems, these concepts remain essential.
FAQs
What is black body radiation in simple terms?
Black body radiation is the heat energy that all objects emit, just because they have a temperature. Even if something looks completely black, it’s still releasing invisible energy in the form of infrared radiation. A black body is a perfect version of this idea—it absorbs all light and heat that hits it and emits the maximum possible radiation for its temperature.
Why is it important in science?
Black body radiation helps scientists understand how heat and light work at a very fundamental level. It led to the discovery of Planck’s law and kicked off the entire field of quantum mechanics. It also helps astronomers measure star temperatures, engineers design thermal sensors, and climate scientists model Earth’s energy balance. Without it, many modern technologies wouldn’t exist.
How is it measured?
Scientists measure black body radiation using tools like spectrometers and thermal cameras. In labs, they use a special device called a black body cavity—a hollow object with a small hole that mimics a perfect black body. By analyzing the emitted radiation’s intensity and wavelength, they can determine the object’s temperature with high accuracy.
What is black body radiation and its characteristics?
Black body radiation is the thermal energy emitted by an object due to its temperature. A black body absorbs all incoming radiation and emits energy across all wavelengths. Its emission depends only on temperature, not material or surface. The radiation follows key physical laws like Planck’s law, Wien’s displacement law, and the Stefan–Boltzmann law.
What are the applications of blackbody radiation?
Black body radiation has many applications in science and engineering. In astrophysics, it helps estimate star temperatures. Thermal cameras use its principles to detect heat in buildings or medical diagnostics. Climate models rely on black body concepts to understand Earth’s heat balance. It’s also crucial in calibrating sensors and studying the cosmic microwave background in cosmology.
What are the characteristics of a perfectly black body?
A perfectly black body has several defining traits:
- Absorbs all incident radiation completely
- Emissivity equals 1 (maximum possible emission)
- Emits radiation only based on temperature, not surface type
- Follows Planck’s law exactly across the entire spectrum
What are the characteristics of a black body radiator?
A black body radiator emits a continuous spectrum of radiation. Its key features include:
- Radiation intensity increases with temperature
- Peak wavelength shifts with temperature (Wien’s law)
- Total emitted energy is proportional to T⁴ (Stefan–Boltzmann law)
- Spectrum follows a predictable shape described by Planck’s law
These traits make black body models essential for temperature measurement and radiation analysis.

4 thoughts on “Planar and Non-Planar Graphs Explained with Circuit Examples”