Contents
Have you ever wondered how scientists visualized the atom before the discovery of the nucleus? Many people assume today’s atomic model has always existed—but that’s far from the truth. In this article on the Tech4Ultra Electrical website, we’ll explore Thomson’s Plum Pudding Model, also known as the Thomson Atomic Model, and understand how it represented a groundbreaking step in atomic theory. Despite its simplicity and eventual rejection, this model played a crucial role in shaping modern physics. By the end, you’ll see why the Plum Pudding Model still matters in the story of scientific progress.
Historical Context and Scientific Foundations
Before diving into Thomson’s Plum Pudding Model, it’s important to understand what scientists actually knew about atoms in the late 1800s. And trust me—it wasn’t much. At that time, the atom was still considered indivisible, a solid sphere as described in Dalton’s atomic theory. No one had any concrete idea about subatomic particles, let alone negative or positive charges inside atoms. Crazy, right?
Everything started to change with experiments involving cathode rays. I remember reading about those old glass tubes and being amazed that such a simple setup—just a sealed tube with low-pressure gas and a high voltage—could reveal something so groundbreaking. When J.J. Thomson applied electric and magnetic fields to the rays, he discovered that they bent, meaning they were made of particles—electrons, as we now know. Even cooler? He managed to calculate the charge-to-mass ratio of these particles. It was the first clear sign that atoms weren’t as solid and indivisible as we’d believed.
Another influence that often gets overlooked is Lord Kelvin’s idea. He had proposed a model where positive charge filled the entire atom, sort of like a soft, positively charged cloud. Thomson took this idea and refined it. In what became known as the Plum Pudding Model, he suggested that negatively charged electrons were embedded within this positive “pudding”—kind of like raisins in a cake.
This combination of experimental data and theoretical inspiration laid the groundwork for the Thomson Atomic Model, the first real step toward understanding the atom’s internal structure.
Read Also: Seebeck Effect: Definition, Formula and Applications
Structure of the Plum Pudding Model
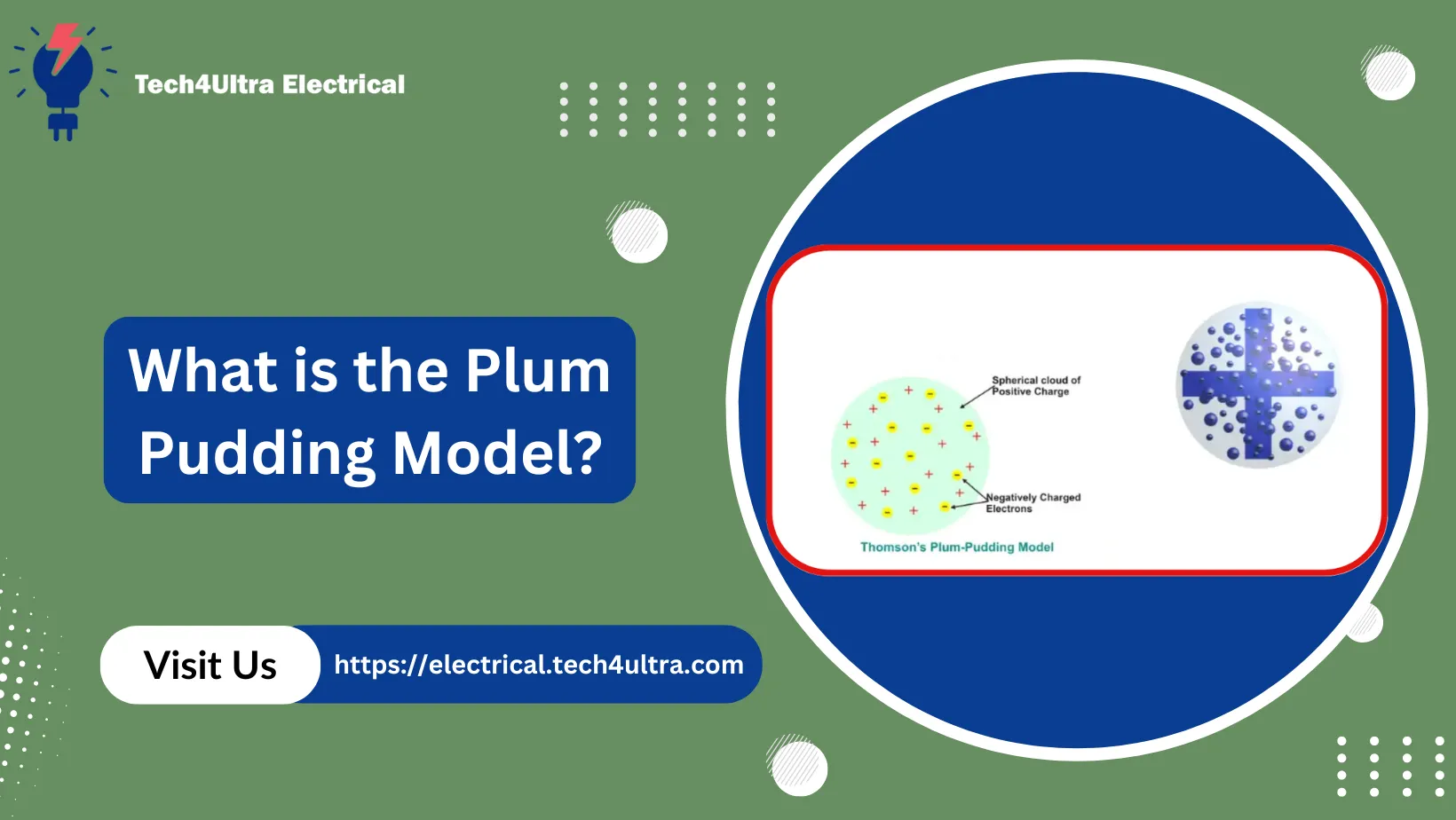
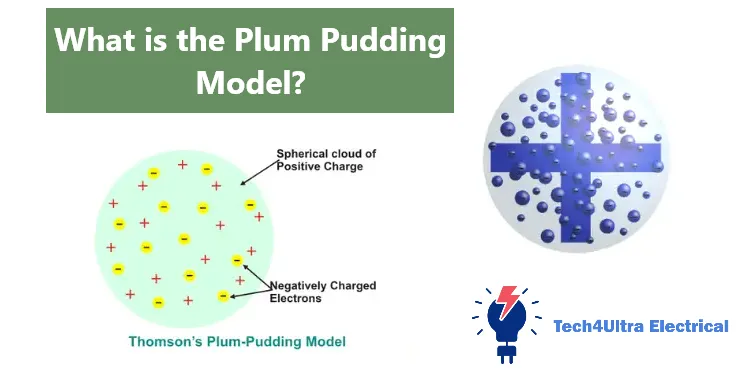
When I first came across the Plum Pudding Model, I honestly thought it sounded like a dessert recipe. But once you get past the funny name, the idea itself is pretty clever—especially considering how little scientists knew about atoms at the time. Thomson’s Plum Pudding Model proposed that the atom was a uniform sphere of positive charge, like a soft “pudding,” with negatively charged electrons scattered throughout it like “plums.” The goal? Keep the atom electrically neutral by balancing those negative electrons with the surrounding positive goo.
It might sound basic today, but back then, this was cutting-edge. Thomson wasn’t just throwing out ideas—he used actual math to back it up. His work with the charge-to-mass ratio of electrons gave him the confidence to estimate how many electrons were needed to balance an atom’s charge. That mathematical backing helped give the Thomson Atomic Model real scientific weight.
Visualize it this way: imagine a big sphere filled with positively charged matter. Now, sprinkle in tiny, negatively charged electrons here and there, evenly spaced so their charges cancel out the positive background. The result is an atom that has no overall electric charge. Pretty neat for its time, right?
Thomson’s model didn’t try to explain everything—like chemical bonding or spectral lines—but it offered the first logical structure that accounted for the existence of electrons without destroying the atom’s stability. And even though it was eventually proven wrong, it was a stepping stone. Without the Plum Pudding Model, we might never have gotten to the atomic models we rely on today.
Early Reception and Support for the Model
At first, the scientific community actually welcomed the Plum Pudding Model. It was one of the first serious attempts to explain how atoms could contain internal structure while still being neutral overall. Many scientists were intrigued. Finally, someone had given them a visual—and mathematical—model that didn’t break the laws of classical physics.
People even tried using Thomson’s Plum Pudding Model to explain chemical bonding. The idea was that atoms might link up by sharing those embedded electrons, forming a kind of electrostatic connection. Sure, it wasn’t a perfect theory, but for a while, it seemed plausible enough.
And because the Thomson Atomic Model didn’t conflict with Newtonian mechanics, it sat comfortably within the classical physics framework of the late 19th and early 20th century. No quantum weirdness yet—just spheres, charges, and symmetry. That made it much easier for other physicists to accept, at least temporarily.
Of course, the model’s weaknesses would become more obvious soon. But in its early days, it was a bold and respected leap forward.
Limitations and Experimental Challenges
As exciting as the Plum Pudding Model was when it first appeared, it didn’t take long before cracks started to show. One of the biggest red flags? It couldn’t explain the hydrogen spectral lines—those super sharp, distinct lines that show up when hydrogen emits light. I remember trying to wrap my head around this as a student and thinking, “Why can’t the electrons in the pudding just vibrate and give off light at those frequencies?” But nope—Thomson’s Plum Pudding Model had no mechanism to account for that kind of behavior. It just didn’t add up.
Then came the real knockout blow: Rutherford’s gold foil experiment. This one changed everything. When Rutherford and his team fired alpha particles at a thin sheet of gold, they expected the particles to pass through almost undisturbed, like bullets through tissue paper. And most did—but a few bounced back at crazy angles. That was shocking. It was as if you fired a cannonball at a piece of tissue and it bounced off.
This single experiment shattered the Thomson Atomic Model. The results clearly showed that atoms weren’t just a smeared-out positive charge with electrons floating inside. Instead, there had to be a small, dense, positively charged center—the atomic nucleus. And that concept? It completely contradicted the plum pudding idea.
In hindsight, the limitations of the model were glaring, but back then, Thomson had no way to predict these outcomes. His model was based on the best data available at the time. Still, once new experimental evidence came in, it was clear the Plum Pudding Model had reached its expiration date.
The Model’s Role in Scientific Evolution
Even though the Plum Pudding Model didn’t stand the test of time, its impact on science was huge. I mean, think about it—it was the first model to say, “Hey, atoms aren’t just solid balls. They’ve got parts.” That shift in thinking opened the door to every atomic theory that came after it.
Without Thomson’s Plum Pudding Model, there might not have been a Rutherford model. And without Rutherford’s nuclear atom, there’s no Bohr model, no orbitals, no quantum leaps. Thomson’s model, flawed as it was, helped scientists start thinking of atoms as structured objects—with internal components and behaviors to study. That was a big leap from just imagining atoms as tiny, indivisible marbles.
More importantly, it set the stage for a deeper dive into atomic theory. It helped bring attention to electrons and forced physicists to ask harder questions. What keeps electrons in place? How do atoms hold together? Why do they emit light in fixed patterns? Those questions pushed science toward quantum mechanics—a field that would soon revolutionize everything we thought we knew.
So yeah, the Thomson Atomic Model might be outdated, but it was a necessary stepping stone. Every great theory builds on the ones that came before it—even the imperfect ones.
Watch Also: Understanding Space Charge: Effects, Laws, and Applications in Modern Electronics
Cultural and Educational Impact
You wouldn’t think a physics model from the early 1900s would show up in places like literature or art—but the Plum Pudding Model actually did. Its quirky name and visual simplicity made it an easy metaphor. I’ve come across poems and essays that use it to describe everything from social structures to philosophical ideas about chaos and order. Turns out, a sphere of gooey charge with floating electrons makes a pretty vivid image!
But its biggest legacy? Education. Thomson’s Plum Pudding Model is still one of the first atomic models students learn about. Why? Because it’s simple. It helps ease you into the complex world of atomic theory without getting overwhelmed by quantum mechanics right away.
It’s also a great teaching tool for showing how science evolves. The model’s rise and fall highlight how scientific theories can be both brilliant and temporary—always ready to be replaced when better data comes along.
Conclusion
The Plum Pudding Model may not have been perfect, but it was a bold attempt to explain something no one had truly seen before. Thomson’s Plum Pudding Model introduced the radical idea that atoms weren’t indivisible—they had internal structure and negative charges.
Even though experiments like Rutherford’s proved it wrong, the Thomson Atomic Model still holds value today. It shows up in every chemistry textbook, not because it’s accurate, but because it’s a critical step in the evolution of atomic theory. It’s a reminder that science grows through trial, error, and courage to challenge the unknown.
Learning about the Plum Pudding Model isn’t just about the past—it’s about understanding how scientific progress really works. And honestly, that lesson is just as important as any equation or discovery.
FAQs
What was the early plum pudding model of the atom?
The early Plum Pudding Model of the atom, proposed by J.J. Thomson, described the atom as a sphere of positive charge with negatively charged electrons embedded throughout—like raisins in a pudding. It aimed to explain atomic neutrality and the presence of electrons after their discovery through cathode ray experiments.
What did the plum pudding model suggest about the atom?
The Plum Pudding Model suggested that atoms are made up of a positively charged “pudding” that holds tiny negative electrons (the “plums”) inside. This model helped early scientists understand how atoms could contain both positive and negative components and still be electrically neutral.
What is the plum pudding model?
The Plum Pudding Model, also called the Thomson Atomic Model, was one of the first scientific attempts to describe the internal structure of the atom. It portrayed the atom as a uniform sphere of positive charge with electrons scattered inside to balance the charge. It was later disproved by Rutherford’s gold foil experiment.
What was Rutherford’s model of the atom?
Rutherford’s model came after the Plum Pudding Model and was based on his gold foil experiment. He proposed that atoms have a small, dense, positively charged nucleus at the center, with electrons orbiting around it—much like planets around the sun. This model replaced Thomson’s and laid the foundation for modern atomic theory.