Contents
What if I told you that one of the 19th century’s most influential inventions is still taught in classrooms today? The Daniell cell—also known as the Daniel cell or Daniell battery—revolutionized electrochemistry by demonstrating how chemical energy can be converted into electrical energy. In this article on the Tech4Ultra Electrical website, you’ll discover everything you need to know about this iconic electrochemical cell, including how it works, its components, and real-world applications. Whether you’re a student, teacher, or just curious about how batteries began, this guide will make the concept of the Daniell cell simple, practical, and unforgettable.
Brief Historical Context and Inventor (John Frederic Daniell)
The Daniell cell was invented in 1836 by English chemist John Frederic Daniell, who sought a more reliable source of electrical power than the unstable voltaic piles of the time. Daniell’s work emerged during a period of rapid development in electromagnetism and electrochemistry, providing scientists with a stable current for experiments and practical use.
Purpose and Significance of the Daniell Cell
The Daniell battery was created to provide a consistent and longer-lasting electric current. Unlike earlier designs, which suffered from quick voltage drops, the Daniel cell solved this by separating the two electrolytes with a porous barrier. Its purpose extended beyond simple lab use; it set the stage for more dependable energy systems and influenced everything from scientific research to early telegraphy.
How It Revolutionized Early Electrochemical Technology
With its stable output, the Daniell cell became a foundational component in early electrical systems. It was widely adopted in physics labs and communication networks, especially for powering telegraphs. Its ability to maintain a consistent voltage over time marked a turning point, leading to the development of more advanced batteries and the broader application of electrical power.
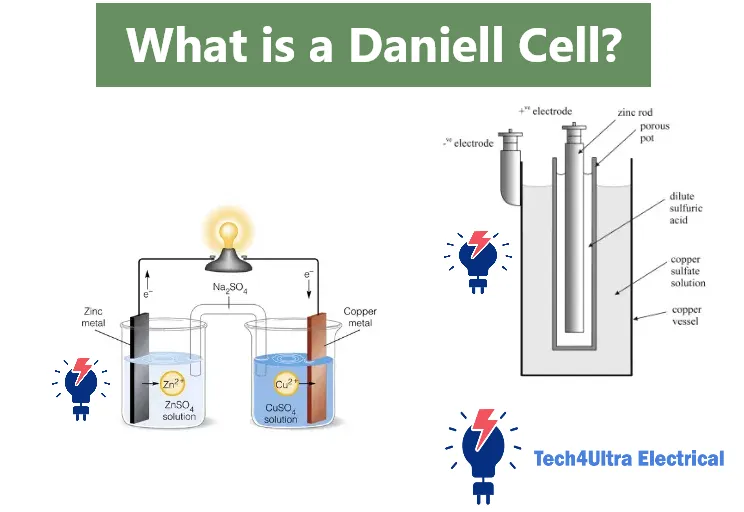
What is a Daniell Cell?
Clear Definition and Classification
A Daniell cell is a type of electrochemical cell specifically classified as both a galvanic and voltaic cell. It generates electrical energy through spontaneous redox (reduction-oxidation) reactions between two different metals immersed in separate electrolyte solutions. These two half-cells are connected by a wire and a salt bridge to complete the circuit.
Explanation of Energy Conversion
The Daniel cell works by converting chemical energy into electrical energy. As electrons flow from the zinc electrode (oxidation) to the copper electrode (reduction), this movement creates an electric current that can be harnessed for external devices. It’s one of the earliest practical examples of harnessing electrochemical reactions for power.
Read Also: Voltaic Cell: Definition, Working, and Examples
Components of a Daniell Cell
Zinc Electrode (Anode)
The zinc electrode in a Daniell cell functions as the anode, where oxidation occurs. This means zinc atoms lose electrons and form zinc ions (Zn2+), which enter the surrounding zinc sulfate solution. The released electrons flow through the external wire toward the cathode. This loss of electrons makes the anode the negative terminal of the cell.
Copper Electrode (Cathode)
The copper electrode serves as the cathode, the site of reduction. In this half-cell, copper ions (Cu2+) from the copper sulfate solution gain electrons arriving through the wire and get deposited as solid copper on the electrode’s surface. This process makes the cathode the positive terminal.
Copper Sulfate and Zinc Sulfate Solutions
Each electrode is immersed in its own electrolyte: the anode in zinc sulfate (ZnSO4) and the cathode in copper sulfate (CuSO4). These solutions supply the necessary metal ions for the redox reactions. The proper concentration of these electrolytes ensures stable voltage and efficient operation of the cell.
Porous Pot or Salt Bridge
To complete the internal circuit, a porous pot or salt bridge is used. This allows ion exchange between the two half-cells while preventing the solutions from mixing directly. It maintains electrical neutrality by allowing anions and cations to migrate appropriately, sustaining continuous electron flow.
Optional Diagram Annotations
Diagram (not shown): Typically includes labels for zinc anode, copper cathode, copper sulfate and zinc sulfate solutions, and a salt bridge or porous barrier between the two half-cells to visualize the flow of ions and electrons.
Construction of Daniell Cell
Step-by-Step Setup
To construct a Daniell cell, start by placing a zinc electrode into a container filled with zinc sulfate solution. This forms the anode half-cell. Next, insert a copper electrode into a separate container with copper sulfate solution to create the cathode half-cell. Connect the two electrodes externally using a conducting wire and insert a salt bridge or porous pot between the solutions to allow ion flow and complete the internal circuit. Electrons flow from zinc to copper, generating electric current.
Porous-Pot Version vs Gravity Cell
In the porous-pot Daniell cell, a porous ceramic vessel containing zinc sulfate is placed inside a larger container with copper sulfate. The porous wall allows ions to pass while keeping solutions from mixing completely. In contrast, the gravity cell uses density differences—copper sulfate at the bottom, zinc sulfate above—without a physical barrier. The layers naturally separate due to gravity, simplifying the construction but requiring careful handling to maintain separation.
Modern Lab Variations
Today’s lab versions of the Daniell battery often use clear beakers and standardized electrodes for better observation and control. Some setups replace the salt bridge with a gel bridge or ion-selective membrane for improved ion flow. These modern tweaks help demonstrate redox reactions clearly while maintaining the core principles of the classic Daniel cell.
Daniell Cell Reactions and Chemistry
Half-Reactions at Anode and Cathode
The core function of the Daniell cell lies in its redox reactions. At the anode (zinc electrode), the reaction is:
Zn(s) → Zn²⁺(aq) + 2e⁻
This is an oxidation reaction—zinc atoms lose electrons and become zinc ions, which dissolve into the zinc sulfate solution.
At the cathode (copper electrode), the reaction is:
Cu²⁺(aq) + 2e⁻ → Cu(s)
This is a reduction reaction—copper ions in the copper sulfate solution gain electrons and deposit as solid copper on the electrode.
Overall Redox Reaction
Combining both half-reactions gives the overall redox equation for the Daniel cell:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
This reaction demonstrates the spontaneous flow of electrons from zinc to copper, which is harnessed as electrical energy in the external circuit.
Role of Salt Bridge in Ion Migration
The salt bridge is crucial for maintaining electrical neutrality in the solutions. As zinc ions accumulate in the anode solution, negatively charged anions (e.g., SO₄²⁻) from the salt bridge move into the anode compartment. Meanwhile, cations (e.g., Na⁺ or K⁺) migrate into the cathode solution to balance the reduction of Cu²⁺ ions. Without this ion migration, the reaction would quickly stop due to charge buildup.
Use of Nernst Equation and EMF Concept
The electromotive force (EMF) of the Daniell battery under standard conditions is approximately 1.10 volts. This can be calculated using the Nernst equation when ion concentrations deviate from standard conditions:
E = E° - (0.059/n) * log(Q)
Where E is the cell potential, E° is the standard EMF, n is the number of electrons transferred, and Q is the reaction quotient. This equation helps predict cell voltage under various chemical conditions, making it a fundamental tool in electrochemistry.
Daniell Cell Voltage and Calculations
Standard Potential (1.1 V)
The standard electromotive force (EMF) of the Daniell cell is approximately 1.10 volts under standard conditions (25°C, 1 M concentration for each ion, and 1 atm pressure). This value is calculated based on the standard electrode potentials of zinc and copper:
Cu²⁺/Cu = +0.34 V, Zn²⁺/Zn = -0.76 V
EMF = 0.34 V – (-0.76 V) = 1.10 V
Factors Affecting EMF
Several factors can influence the voltage output of a Daniel cell, including:
- Ion concentrations in the electrolytes
- Temperature variations
- Electrode surface area and purity
- Internal resistance and degradation of the salt bridge
Application of Nernst Equation
To calculate the actual cell potential under non-standard conditions, the Nernst equation is used:
E = E° - (0.059/n) * log([Zn²⁺]/[Cu²⁺])
This allows chemists to determine how changing concentrations of copper and zinc ions affect the EMF of the Daniell battery, making it a powerful predictive tool in electrochemical studies.
Comparison: Daniell Cell vs Voltaic Cell
Differences in Design, Stability, Safety
While both the Daniell cell and the voltaic cell are galvanic cells that generate electricity through spontaneous redox reactions, their designs differ significantly. The original Voltaic cell, invented by Alessandro Volta, used alternating layers of zinc and copper discs soaked in saltwater-soaked cloth. It was simple but suffered from poor voltage stability and rapid degradation.
The Daniell battery, on the other hand, introduced separated electrolyte compartments connected by a salt bridge or porous pot, resulting in a more stable and longer-lasting output. It also reduced internal resistance and allowed safer, more controlled reactions—making it suitable for scientific and communication applications.
Problem of Hydrogen Bubbles Solved
A key issue with the Voltaic cell was the accumulation of hydrogen bubbles on the copper electrode, which increased resistance and interrupted current flow. The Daniell cell solved this by replacing hydrogen evolution with copper ion reduction, producing solid copper instead of gas. This innovation greatly improved reliability and efficiency.
Daniell Cell in History and Applications
Use in Telegraphs and Electrotyping
In the 19th century, the Daniell cell played a crucial role in powering telegraphs, especially during the expansion of railway communication. Its stable voltage and long-lasting performance made it the go-to energy source for long-distance signaling. In addition, the Daniell battery was widely used in electrotyping—a process that involved creating metal printing plates by electroplating, essential in the printing industry for reproducing images and text with precision.
Basis of Early Voltage Standards
The Daniel cell was so reliable that it became a standard reference for electrical potential in early scientific research. Its EMF of approximately 1.1 volts was consistent enough to serve as a benchmark for calibrating voltmeters and standardizing electrical measurements across laboratories and institutions.
Importance in Educational Demonstrations
Even today, the Daniell cell remains a favorite in chemistry and physics classrooms. Its simplicity and visible redox reactions make it an ideal tool for teaching students about oxidation, reduction, and the fundamentals of electrochemical cells. Demonstrating how chemical reactions produce electricity helps bridge theory and practice in a tangible, engaging way.
Variants and Developments
Gravity Cell (Crowfoot Cell)
One of the most well-known variants of the Daniell cell is the gravity cell, often called the crowfoot cell due to the shape of its copper electrode. It operates without a porous pot or salt bridge, relying instead on the natural separation of copper sulfate (denser) and zinc sulfate (lighter) solutions. This made it cheaper and easier to maintain, ideal for long-running systems like telephone exchanges.
Bird’s Cell
The Bird’s cell was a modification designed to enhance durability and reduce maintenance. It featured improved materials and electrode configuration for more stable performance over time, and it was popular in marine and military applications.
Daniell-Type Cells in Research
Though no longer common in practical energy systems, Daniell-type cells are still used in electrochemistry research and academic experiments. They help study reaction kinetics, ion transport, and electrode behavior under controlled conditions, maintaining their role as a valuable educational and scientific tool.
Advantages and Limitations
Advantages of the Daniell Cell
The Daniell cell offers several key advantages. Most notably, it provides a stable and consistent voltage output, making it ideal for long-term applications like telegraphy and lab experiments. Unlike early voltaic cells, it does not suffer from polarization, thanks to the copper ion reduction reaction that replaces hydrogen evolution. This ensures a steady flow of current without interruptions.
Limitations of the Daniell Cell
Despite its strengths, the Daniel cell comes with limitations. Its limited lifespan means the electrodes and solutions must be replaced periodically. Additionally, the use of corrosive solutions like copper sulfate and zinc sulfate requires careful handling and proper disposal, especially in modern labs. It also lacks portability and energy density compared to today’s compact battery technologies, making it unsuitable for mobile or high-power applications.
Watch Also: Types of Battery: Primary, Secondary, and More
Modern Relevance and Legacy
Role in Battery Evolution
The Daniell cell laid the groundwork for modern battery technology. Its design introduced the concept of separated half-cells and ionic flow control, principles that still underpin today’s lithium-ion and alkaline batteries. By demonstrating how chemical reactions could reliably generate electricity, the Daniell battery shaped the path for portable power solutions we now take for granted.
Importance in Chemistry Education
In classrooms around the world, the Daniel cell remains a staple for teaching electrochemistry. It visually and practically illustrates redox reactions, electron flow, and energy conversion—making complex theory easier to understand and apply for students of all levels.
Conclusion
The Daniell cell, also known as the Daniell battery or Daniel cell, is a foundational electrochemical cell that transforms chemical energy into electrical energy through a redox reaction. Invented by John Frederic Daniell, it introduced the concept of separated electrolytes, reducing polarization and improving voltage stability. Its components—zinc and copper electrodes, sulfate solutions, and a salt bridge—formed the blueprint for modern battery systems. Beyond its historical impact, the Daniell cell continues to play a vital role in chemistry education, offering a clear, hands-on understanding of electrochemical principles still relevant in today’s energy technologies.
FAQs
What is the Daniell cell?
The Daniell cell is a type of galvanic cell that converts chemical energy into electrical energy using two half-cells: one with a zinc electrode in zinc sulfate and the other with a copper electrode in copper sulfate. It produces a stable voltage of about 1.1 volts and was a major advancement over earlier voltaic cells due to its improved efficiency and reliability.
What is the construction of the Daniel cell?
The Daniel cell is constructed using two separate containers or compartments. One contains a zinc electrode in a zinc sulfate solution (anode), and the other contains a copper electrode in a copper sulfate solution (cathode). These are connected via a salt bridge or a porous pot that allows ion exchange while keeping the solutions mostly separated.
How to build a Daniell cell?
To build a Daniell cell, place a zinc strip in a zinc sulfate solution and a copper strip in a copper sulfate solution. Connect both electrodes with a conductive wire and use a salt bridge filled with a neutral salt like KNO₃ to link the two solutions. This setup allows electrons to flow from zinc to copper, generating electricity.
What is the construction and working of a galvanic cell?
A galvanic cell consists of two half-cells with different metals in electrolyte solutions. One metal acts as the anode (oxidation site), and the other as the cathode (reduction site). They are connected by a wire for electron flow and a salt bridge for ion flow. The Daniell cell is a classic example of this, converting spontaneous redox reactions into usable electrical energy.

1 thought on “Sinusoidal Wave Signal: Definition and Examples”