Contents
Did you know that atoms were once believed to be indivisible solid spheres? That misconception ruled science until Ernest Rutherford stepped in. In this article on the Tech4Ultra Electrical website, you’ll discover how the Rutherford Atomic Model shattered that outdated view and laid the foundation for modern atomic theory. By exploring the key concepts of the Rutherford model, you’ll gain a clearer understanding of atomic structure and why this breakthrough changed the course of physics forever.
For centuries, scientists have been fascinated by the question: what is matter made of at its core? The journey to answer that has taken us through several stages, each marked by groundbreaking discoveries that reshaped our understanding of the atom. From ancient philosophical ideas to modern physics, atomic theory has undergone dramatic evolution.
It all started with John Dalton in the early 1800s, who proposed that matter is composed of small, indivisible particles called atoms. His model, while revolutionary for its time, pictured the atom as a solid, indestructible sphere—something like a tiny, dense billiard ball.
Then came J.J. Thomson, who discovered the electron in 1897. His “plum pudding model” suggested that atoms were not indivisible but instead contained negatively charged particles embedded in a positively charged “pudding.” This idea, although flawed, introduced the concept of internal structure within the atom.
However, the real shift came with Rutherford’s atomic model. Through his famous gold foil experiment, Ernest Rutherford revealed that atoms are mostly empty space, with a dense, positively charged nucleus at the center. The Rutherford model disproved the plum pudding theory and laid the foundation for the nuclear model of the atom we study today.
Who Was Ernest Rutherford?
Ernest Rutherford, often called the father of nuclear physics, was a New Zealand-born British physicist whose work transformed our understanding of the atomic world. Born in 1871, Rutherford displayed a deep curiosity for science early on, eventually earning a scholarship to study at Cambridge University. There, he worked under J.J. Thomson before embarking on his own groundbreaking experiments.
Rutherford’s contributions went far beyond just the Rutherford model. He discovered the concept of radioactive half-life, identified alpha and beta radiation, and even succeeded in splitting the atom—an achievement that laid the groundwork for modern nuclear physics. His development of Rutherford’s atomic model wasn’t just a scientific breakthrough; it marked the beginning of a new era in understanding matter and energy at the most fundamental level.
Read Also: Plum Pudding Model Explained: Thomson’s Early Atomic Theory
The Gold Foil Experiment (Geiger–Marsden Experiment)
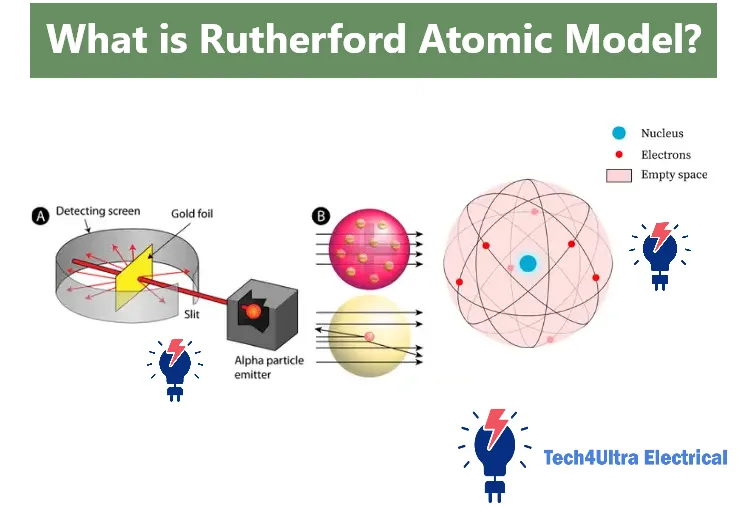
One of the most defining moments in atomic science was the gold foil experiment, conducted by Hans Geiger and Ernest Marsden under the guidance of Ernest Rutherford in 1909. This experiment provided the foundation for what would become Rutherford’s atomic model, radically altering how scientists viewed the structure of the atom.
The experimental setup was surprisingly simple yet ingenious. A thin sheet of gold foil—just a few atoms thick—was bombarded with a stream of alpha particles, which are positively charged and relatively massive. Around the foil, a fluorescent screen coated with zinc sulfide was used to detect where the particles struck. Each impact created a tiny flash of light, observed using a microscope in a darkened room.
The expectations at the time were based on the plum pudding model: the alpha particles should have passed straight through the foil with minimal deflection. However, what Geiger and Marsden observed shocked the scientific community.
Most alpha particles did pass through the gold foil, confirming that atoms are mostly empty space. But—crucially—a small fraction of the particles were deflected at large angles, and a very few even bounced straight back. This unexpected scattering phenomenon could not be explained by Thomson’s model.
It was this surprising result that led Rutherford to propose the Rutherford model, where the atom consists of a dense, positively charged nucleus at the center, with electrons orbiting around it—much like planets around the sun. The experiment remains a cornerstone of nuclear physics and a perfect example of how experimental data can overturn long-standing theories.
Postulates of Rutherford’s Atomic Model
After analyzing the results of the gold foil experiment, Ernest Rutherford proposed a new structure for the atom that became known as Rutherford’s atomic model. This model marked a major departure from earlier theories by introducing a nuclear-centered view of the atom. Below are the main postulates that defined the Rutherford model:
- Nucleus as the core: At the center of every atom lies a dense, compact nucleus. This nucleus carries most of the atom’s mass and holds a positive electric charge. The concept of the nucleus was revolutionary, replacing the idea of a uniform atomic distribution.
- Electrons orbiting the nucleus: Rutherford proposed that negatively charged electrons revolve around the nucleus in specific orbits. Though he didn’t explain why they don’t spiral inward (a problem later addressed by Bohr), this idea was a major leap in atomic theory.
- Atom mostly empty space: Since most alpha particles passed through the gold foil unimpeded, Rutherford concluded that atoms are primarily empty space. This explained why particles could move through matter without significant interaction.
- Positive charge concentration: Unlike the plum pudding model, where positive charge was spread throughout, the Rutherford model concentrated it in the nucleus. This explained the strong repulsive forces that deflected some alpha particles at large angles.
These postulates transformed atomic theory and laid the groundwork for the development of quantum mechanics. Though not without limitations, Rutherford’s atomic model was a pivotal step in unraveling the mysteries of atomic structure.
The Nuclear Model of the Atom
The introduction of the Rutherford model marked a turning point in atomic theory. By placing a dense nucleus at the center of the atom, Rutherford’s atomic model redefined our understanding of matter at its most fundamental level. It moved away from the idea of a uniformly distributed atom to a structured, dynamic model with clear internal organization.
One of the most important shifts was the realization that most of the atom’s mass and all its positive charge are concentrated in the nucleus. The electrons, although tiny in mass, orbit this nucleus and account for the atom’s volume. This explained why alpha particles could mostly pass through matter unimpeded, while a few were sharply deflected.
Compared to Thomson’s plum pudding model—where electrons were scattered within a diffuse cloud of positive charge—the Rutherford model was both simpler and more accurate. It provided a clear explanation for the scattering of alpha particles and gave the first glimpse into the atom’s true architecture: a small, dense center surrounded by empty space and orbiting electrons.
Limitations of Rutherford’s Atomic Model
While Rutherford’s atomic model was a monumental leap forward in understanding atomic structure, it wasn’t without flaws. Despite its successes in explaining the existence of a nucleus and the scattering of alpha particles, the Rutherford model struggled to address several critical issues that later paved the way for quantum theory.
One of the biggest limitations was the model’s reliance on classical mechanics. According to classical electromagnetic theory, electrons orbiting a nucleus should constantly emit radiation. This would cause them to lose energy, spiral inward, and eventually crash into the nucleus—leading to atomic collapse. Yet, atoms are stable, which posed a major contradiction.
Another issue was the model’s failure to explain discrete electron energy levels. Spectral analysis shows that atoms emit light at specific wavelengths, implying that electrons occupy fixed energy states. However, Rutherford’s atomic model couldn’t account for why electrons didn’t emit continuous radiation or why they didn’t fall into the nucleus under the influence of attractive forces.
In short, while the Rutherford model was a crucial step in atomic theory, it couldn’t fully explain atomic behavior at the microscopic level. These gaps led to the development of quantum mechanics and the refinement of atomic models, most notably by Niels Bohr.
Rutherford vs. Bohr: Key Differences
Although Rutherford’s atomic model introduced the idea of a nucleus and orbiting electrons, it couldn’t explain why atoms were stable or why electrons didn’t radiate energy and collapse into the nucleus. This gap paved the way for Niels Bohr to refine the model in 1913, combining Rutherford’s structure with quantum theory.
Bohr’s model preserved the nuclear core introduced in the Rutherford model but added one crucial idea: electrons can only occupy certain energy levels. Instead of spiraling inward, electrons move in fixed orbits with quantized energy, and they only emit or absorb energy when jumping between these levels. This concept perfectly explained the discrete spectral lines observed in hydrogen.
In summary, the main difference lies in stability and energy quantization. Bohr gave the atom a quantum structure, something Rutherford’s atomic model lacked, making it a more complete and accurate representation of atomic behavior.
Watch Also: Electron Configuration Explained: Rules, Examples, and Notation
Impact and Legacy in Modern Atomic Physics
Despite its limitations, Rutherford’s atomic model laid the essential groundwork for all future atomic theories. By introducing the nucleus and proposing that atoms are mostly empty space, the Rutherford model redefined how scientists approached the structure of matter. It shifted the scientific perspective from uniform atomic structures to one centered around a dense core and orbiting electrons.
This model became the cornerstone for future developments in nuclear physics and quantum mechanics. It directly influenced Niels Bohr’s refinements and ultimately paved the way for the discovery of protons, neutrons, and the development of quantum field theory. Rutherford’s ideas even helped guide early experiments in particle physics and nuclear reactions, including the eventual splitting of the atom.
In essence, Rutherford’s atomic model not only challenged existing beliefs—it sparked an entire era of scientific exploration that still shapes modern physics today.
Conclusion
Rutherford’s atomic model marked a turning point in our understanding of atomic structure. By introducing the nucleus and redefining the atom as mostly empty space, the Rutherford model set the foundation for modern physics. Although it couldn’t answer every question, it challenged long-held theories and inspired future breakthroughs in quantum mechanics and nuclear science. Today, it remains a powerful example of how experimental evidence can reshape scientific thinking—and how one model can become the stepping stone to an entirely new era of discovery.
FAQs
What is the Rutherford atomic model in simple terms?
Rutherford’s atomic model describes the atom as having a tiny, dense, positively charged center called the nucleus. Electrons orbit this nucleus like planets around the sun. Most of the atom is empty space, allowing particles to pass through with little resistance. It was a major shift from previous models that viewed the atom as a solid or uniformly charged structure.
Why did Rutherford’s model fail?
While the Rutherford model introduced the nucleus and explained atomic scattering, it couldn’t explain why electrons didn’t lose energy and spiral into the nucleus. Classical physics suggested that orbiting electrons should emit radiation and collapse. It also failed to account for the fixed energy levels seen in atomic spectra, which led to the development of quantum models like Bohr’s.
What did the gold foil experiment prove?
The gold foil experiment showed that atoms are mostly empty space with a dense central core—the nucleus. When alpha particles were shot at thin gold foil, most passed through, but a few were deflected sharply. This proved the existence of the nucleus and invalidated the plum pudding model, forming the basis of Rutherford’s atomic model.
What is Rutherford’s atomic model theory?
Rutherford’s atomic model theory proposed that an atom consists of a dense, positively charged nucleus surrounded by electrons moving in orbits. Most of the atom’s volume is empty space. This was a major advancement over previous models, introducing the concept of the nucleus and a structured atomic interior.
What was the conclusion of Rutherford’s model of the atom?
The main conclusion of the Rutherford model was that atoms are not solid masses but have a tiny nucleus at their center, which contains most of the mass and positive charge. Electrons orbit this nucleus, and the vast majority of the atom is empty space. This explained why most alpha particles passed through gold foil with minimal deflection.
How did Rutherford discover the model?
Rutherford developed his atomic model after analyzing results from the gold foil experiment, conducted with Geiger and Marsden. When alpha particles were directed at a thin sheet of gold, most passed through, but some were deflected at sharp angles. This unexpected behavior led Rutherford to propose that atoms have a central nucleus, forming the basis of the Rutherford model.
Why is the Rutherford atomic model important?
The Rutherford atomic model is important because it introduced the nucleus, changing how we understand atomic structure. It corrected the flaws in previous models and set the stage for quantum mechanics and future discoveries in nuclear and particle physics. It remains a cornerstone in the history of atomic theory.
What is Rutherford best known for?
Ernest Rutherford is best known for developing Rutherford’s atomic model and discovering the atomic nucleus through the gold foil experiment. He is also credited with laying the foundation for nuclear physics by classifying types of radiation and being the first to split the atom.
Why is the atomic model so important?
The atomic model is crucial because it helps us understand the structure and behavior of matter. Each model, including the Rutherford model, provided a stepping stone toward more accurate scientific theories, enabling advances in chemistry, physics, and technology.
What is the most important atomic theory?
One of the most important atomic theories is the quantum mechanical model, which describes electrons in probabilistic orbitals rather than fixed paths. However, the Rutherford model remains vital as a historical and conceptual breakthrough in identifying the nucleus.
What is the aim of the atomic model?
The aim of any atomic model is to represent how atoms are structured and how they behave during chemical reactions and physical interactions. Rutherford’s atomic model aimed to explain atomic structure based on experimental results, particularly the gold foil experiment.
What atomic model is used today?
Today, scientists use the quantum mechanical model of the atom. It builds on and refines concepts from earlier models like the Rutherford model, providing the most accurate understanding of electron behavior and atomic interactions through wave mechanics and probability.
What is the most famous atomic model?
The Bohr model is often considered the most famous atomic model due to its successful explanation of hydrogen’s spectral lines. However, Rutherford’s atomic model is equally iconic for introducing the nucleus and fundamentally changing atomic theory.
What is the difference between Dalton and Rutherford model of the atom?
Dalton’s model described atoms as indivisible solid spheres, with no internal structure. In contrast, Rutherford’s atomic model introduced a central nucleus and orbiting electrons, based on experimental evidence from the gold foil experiment—making it far more detailed and accurate.
How did Bohr’s model differ from Rutherford’s?
While the Rutherford model proposed electrons orbiting a nucleus, it didn’t explain atomic stability. Bohr improved on this by introducing quantized orbits—electrons could only occupy specific energy levels, preventing them from spiraling into the nucleus and better matching observed atomic spectra.